2,3,5,6-Tetramethyl pyrazine did not pop up overnight. This compound goes back to the deep roots of organic chemistry, with researchers first bumping into the pyrazine family over a century ago. Early studies pulled these nitrogen-containing rings out of all sorts of fermentation broths and roasted foods. Pyrazines give those flavors their signature nuttiness and roast. Scientists started isolating individual members, and before too long, they identified the tetramethyl variant in fermented soy products and cocoa. The food industry soon took notice, hungry for authentic flavors and aromas. Since then, companies and academics alike have profiled tetramethyl pyrazine in everything from taste tests to cellular experiments. My own time in the lab showed how persistent chemists can be, endlessly tweaking conditions to squeeze out better yields or cleaner isolates, hinting at both the stubbornness of the molecule and the creativity of those who work with it.
2,3,5,6-Tetramethyl pyrazine, also called ligustrazine, pops up as a pale yellow to light brown crystal, sometimes described as holding a cocoa-like, nutty scent that tips off the senses long before any molecule gets under a microscope. Because it comes both in nature and through chemical synthesis, manufacturers have filled warehouses with it for everyone from food technologists to pharmaceutical researchers. Demand grows as people chase flavor authenticity or explore traditional medicine's chemical underpinnings. I’ve seen smaller flavor houses order it by the barrel, eager to punch up chocolate and baked-goods profiles, while pharmaceutical suppliers keep it on hand for labs exploring everything from heart-protective potentials to anti-tumor agents.
This compound, C8H12N2, carries a molecular weight around 136.20 g/mol. It usually melts in the range of 78–80°C and boils at temperatures close to 225°C. Solubility sits at the crossroads: it easily dissolves in alcohol and ether yet avoids water. The aroma escapes easily, and even at low concentrations you’ll catch that familiar roasted, earthy hint. Its appearance can fool newcomers—what looks like just another pale crystalline powder turns out, under the right conditions, to be a heady presence in the room. These characteristics matter whether you are blending flavors or handling it in a research facility, since volatility not only decides scent release, but also safety protocols.
Manufacturers generally label batches with clarity regarding purity, often aiming above 98%. Labels note physical state, appearance, melting and boiling points, storage directions, and relevant hazard statements under local or international chemical safety regimes. Safety Data Sheets (SDS) accompany every drum, listing all identifiers, hazard pictograms, and exposure safe handling advice. In my last job, even flavorists running a bench-top test wore gloves and goggles, and the containers never left the designated chemical storage. Nothing says “pay attention” quite like the international exclamation mark for skin or eye irritants. Every label needs to tell you not just what it is, but what happens if it touches your skin or you breathe too much in.
Industrial synthesis starts by methylating pyrazine under carefully controlled conditions, juggling raw materials like methyl iodide or methyl sulfate with base and solvent, then purifying the result by distillation or recrystallization. Traditionalists, often in Asia, still coax it from fermented beans or roots, but that’s a slow road compared to modern chemical reactors. Commercial producers focus on two things: product yield and purity. They fine-tune pH, temperature, and reaction time, balancing costs with demand from food and pharmaceutical customers. During graduate school, purification often proved trickier than reaction—removing those last trace impurities or side products calls for sharp eyes and patience.
Pyrazines like to hold onto their methyl groups, but you can coax them into further transformations with just the right conditions. Scientists add or swap out groups, testing for new aromas, or in pharmaceutical contexts, probing for biological activity. It’s fascinating how a little tweak to the ring, an extra methyl here or a substituent there, can push the aroma from roasted peanut to rye bread, or switch a molecule from harmless to potential medication. This is a playground for the flavor chemist and drug designer alike. Whole patents hang on just where those extra atoms latch onto the ring.
Besides its systematic name, this compound answers to ligustrazine, TMP, tetramethylpyrazine, and sometimes 2,3,5,6-tetramethyl-pyrazin. Food scientists lean on the shorter forms, while botanists or pharmacologists use the more formal ligustrazine, hearkening back to its identification in the herb Ligusticum wallichii. Some suppliers brand it with catchy trade names for flavor blends or pharmaceutical ingredients. Such a range of synonyms can confuse newcomers—I once lost hours hunting through literature because someone used a little-known synonym instead of the name everyone in the flavor industry prefers.
Handling ligustrazine means respecting its volatility and the risk of irritation, using gloves, lab coats, and well-ventilated hoods. Storage needs a cool, dry, clearly labeled spot. Regulatory bodies like OSHA, ECHA, and China’s NMPA issue handling and exposure guidelines. Any accidental spillage calls for immediate cleanup and proper disposal—no pouring down sinks or casual sweeping up. Emergency procedures line the walls in every flavor and pharma facility I’ve walked into. And still, as with too many chemicals, surprises happen, so ongoing training never ends.
In the world of food, 2,3,5,6-tetramethyl pyrazine gives chocolates, baked goods, roasted nuts, coffees, and even popcorn that extra authentic note prized by brands chasing repeat business. The pharmaceutical field leans hard on its role in traditional Chinese medicine, using it as a lead compound in drugs for cardiovascular and cerebrovascular disorders. Technical specialists are exploring how its neuroprotective effects might benefit stroke and dementia patients. As a flavor enhancer, it sits on the shelf beside vanillin and benzaldehyde, key to giving processed foods the depth people expect but seldom realize comes from a few molecules added at trace levels.
Academic labs and industry R&D teams run parallel races. Flavor developers screen derivatives for safer or longer-lasting effects; the pharmaceutical sector dissects modes of action at the molecular and biochemical levels. Recent years brought breakthroughs where combination therapies with ligustrazine improve recovery rates after ischemic events. Research journals fill up with animal studies and even small-scale clinical trials, sparking hope for new treatments. When I spent a stint reviewing literature for a biotech startup, I was struck by the packed reference lists—dozens of research groups chasing down every known and unknown action ligustrazine might have, especially in vascular and brain health.
Toxicological testing runs deep. Traditional use suggested safety, but systematic studies exposed some mild cytotoxic effects at higher dosages. Most toxicity appears in vitro or with excessive animal exposure, with symptoms involving liver or nervous system stress. Regulatory bodies keep reviewing levels allowed in foods and drugs based on new data. Chronic exposure studies look for long-term, low-level risks, so labeling and recommended uses evolve with each new report. I’ve read both the hopeful and cautionary headlines in toxicology digests—results keep shifting as research methods sharpen and dose-response data gets clearer.
Looking forward, demand keeps rising on two fronts: cleaner, more authentic food flavors and evidence-backed natural supplements. Plant-based food producers, facing fierce competition for market share, aim to blend the compound into meat alternatives or energy bars. In pharmaceuticals, every new insight into neuroprotective or anti-inflammatory action creates a freshly funded clinical trial. The biggest opportunities lie at the intersection of synthetic biology and green chemistry—companies are already engineering microbes to churn out ligustrazine from cheap sugars, which could slash costs and reduce waste. Regulatory hurdles remain a challenge, especially outside of Asia, but global acceptance seems likely as studies continue to show consistent benefits and generally good safety margins.
If you’ve ever noticed the comforting smell that fills a room as bread bakes or coffee roasts, you’ve brushed up against the world of pyrazines. Among these, 2,3,5,6-Tetramethyl Pyrazine (sometimes called ligustrazine) offers a powerful punch in the flavor industry. This molecule shows up in cocoa, nuts, and roasted grains, helping deliver that deep, nutty aroma that triggers cravings. Companies hunt for ingredients that can turn bland products into irresistible ones, and Tetramethyl Pyrazine fills that role. Food scientists can recreate a roasted, savory taste in processed snacks, chocolate, or meat alternatives without fire or smoke—just a few sprinkles of this compound.
Traditional Chinese medicine stretches back thousands of years, but not all remedies from these roots get embraced by scientists. Tetramethyl Pyrazine stands out because research has supported some of its benefits. Hospitals in China and across Asia use it to help patients with strokes or heart problems. Studies have looked into how this compound can help thin the blood or expand blood vessels, making it easier for oxygen to reach damaged areas.
A few years ago, I volunteered in a senior home in a city with a large Chinese community. Friends would share how their doctors prescribed medicine containing ligustrazine extract for blood circulation or to help people recovering from minor strokes. As a writer who likes to double-check, I looked up peer-reviewed research. There’s evidence it can lower inflammation and reduce damage from blocked blood vessels, though scientists in the West push for larger studies before calling it a miracle medicine.
Chemists often work behind the scenes, but their work affects people’s daily lives. At a flavor house, technicians blend chemicals to mimic everything from bacon to hazelnuts. Tetramethyl Pyrazine sits on the shelf as one of the go-to choices for intensifying flavors. The food industry looks for consistency—having a stable, pure source cuts down waste and lets chefs depend on flavors not changing from batch to batch.
In the lab, this molecule acts as a building block for other chemicals. Universities and big pharmaceutical firms try to tweak the molecule a bit, hoping to discover drugs that can target new illnesses. Throw in the compound and see what sticks—that’s how innovation starts. Sometimes the best new medicines come from molecules people once tasted in their morning toast.
Even as people sing its praises, there are challenges. Use a powerful chemical like this and you have to think about exposure, allergies, and regulation. Food safety authorities watch for anyone overusing flavoring agents, and health agencies want evidence that dosage is safe for daily use. If I learned anything from talking to food safety experts, it’s the need for clear labeling. It’s tough enough for folks with sensitivities to avoid triggers, and hidden ingredients only make it harder.
Beyond food, companies selling herbal remedies must step up their testing game. Years of anecdotal evidence mean something, but mixing tradition with modern research keeps everyone safer. If the West picks up on the potential benefits, expect a rush of new studies, public scrutiny, and a push for more rigorous clinical trials.
Tetramethyl Pyrazine’s journey covers a lot of territory—from toast to hospital wards. The lesson here is that a compound can feed you, heal you, or sometimes complicate things further. With smart oversight and open conversation about risks and benefits, people get the best from this little molecule without stumbling into trouble.
In food labs and kitchens, folks use all sorts of ingredients to tweak our snacks and meals. 2,3,5,6-Tetramethyl pyrazine pops up sometimes, especially in flavors that remind us of roasted nuts, baked bread, or chocolate. This compound gets attention for that deep, toasty aroma, but safety questions keep circling. Who really knows if something with a complicated name like that should end up in our bellies?
2,3,5,6-Tetramethyl pyrazine comes from a group of natural chemicals that build up during the Maillard reaction. If you think about that rich smell from fresh coffee beans or toast, much of it traces back to these compounds—pyrazines among them. Food scientists figured out long ago that a sprinkle of these flavor molecules can bump up the indulgence in all sorts of snacks, soups, and seasonings. It’s not unusual for producers to add small amounts, hoping for a bigger flavor punch.
People want assurances before biting into anything new. Research teams and regulatory agencies ran toxicology tests on this molecule, mostly sticking to rodents and in vitro setups. The current data show little sign of harm at the amounts typically used in food. Both the U.S. Flavor and Extract Manufacturers Association (FEMA) and the European Food Safety Authority (EFSA) reviewed it for flavor purposes. No significant health worries showed up for humans at the concentrations allowed in snacks or drinks.
These agencies rely on conservative safety margins. They look at how much a usual person eats in a day, then slash that amount by huge factors to set safe limits. Even with repeated exposure, there’s no strong sign of toxicity, cancer risk, or anything that points to organ damage for humans. Most folks eat so little that the numbers barely register compared to much riskier lifestyle choices, like burning food or eating highly processed meats every day.
Even with all the studies, not everyone trusts laboratory data alone. Real-life diets can involve way more mixing and matching than what a rat in a cage eats. Some people worry about all these additives coming together over years, stacking up inside us. Others just bristle at scientific names, remembering scares from the past such as artificial colorings or trans fats, which once got a green light before problems showed up.
What I’ve noticed is that science moves faster than public comfort. Most of us want to know our food isn’t some risky experiment. As a parent, I’d rather see more independent testing, not just industry-backed safety checks. If scientists can’t answer tough questions about how everything interacts in the long term, a little extra caution feels justified.
The safest way to use this and other newish additives? Push for more research in actual food, with honest, long-term observation. If you cook at home, stick to flavors from whole ingredients. Mostly, food businesses should stay transparent about ingredients and offer clear details about their testing process. Share what researchers already know and where they admit gaps. Let people make their own choices, with real data close at hand.
You see stories about food scandals, fake drugs, and chemical recalls far too often. It all boils down to trust—trust in what’s written on the label, trust in who’s making the stuff, and trust in the purity. If you’ve ever worked in a lab or in the food business, you know that 2,3,5,6-Tetramethyl Pyrazine is a big deal. People reach for it to get that punchy, roasted aroma—nutty, chocolatey, like bread straight from the oven. Suppliers throw around numbers like “99% pure” or even higher, but what does that really mean? And why should anyone care?
A purity of 99% or better sounds impressive. To the casual buyer, it suggests right-on-the-money composition. There’s a catch, though. A certificate can show high purity, but the actual product on your pallet could tell another story if you bothered to run your own gas chromatography test. I’ve seen suppliers fudge their purity numbers to undercut competitors. If you aren’t checking, you’re often just gambling with your money and your reputation.
Stories pop up from time to time: a flavored drink comes off strange. Or the new snack formulation tastes just shy of right. There’s only a sliver of an off-note, but that’s enough to throw out a whole batch. Nine times out of ten, the finger points straight back to the raw ingredients. Maybe the 2,3,5,6-Tetramethyl Pyrazine carried traces of solvents left over from manufacturing, or leftover isomers that tweak the flavor. These aren’t always things you spot during routine checks, especially when management pushes for lower costs and faster turnaround.
Purity isn’t only about taste. In pharmaceuticals, even tiny contaminants can spell trouble. I remember a run-in with a batch that should have read 99.5%, according to paperwork. After a couple of headaches with batch performance, we ran a deep analysis. Turned out the level hovered closer to 96%, and the rest carried micro-levels of unlisted byproducts. A headache on the production line, and a lot of wasted material.
Some contaminants might not seem dangerous upfront, yet they can cause allergies or build up in body tissue over time. In one reported case, lower-purity lots were traced back to a spike in consumer complaints, ending with authorities pulling products from shelves. The loss in public trust often proves much pricier than the savings from a cheap supplier.
Blindly trusting certificates has never worked for me. I stick to suppliers that allow regular third-party purity checks. Paying a bit more for full transparency ends up saving money and headache down the line. I suggest running bimonthly or quarterly spot-checks—not just on new suppliers, but even the ones you think you know.
In the food and flavor industry, ignoring small fluctuations in raw ingredient purity can ruin months of research and new product launches. Tighter supplier partnerships, open communication, and an established in-house quality check do more than cover your bases; they keep quality consistent and the end user safe. I speak from experience: a single extra step in raw material verification goes far in keeping both the customer and the business side happy.
Whether you’re working with a chemical lab, a food company, or just trying to make sense of safety sheets, handling compounds like 2,3,5,6-Tetramethyl Pyrazine puts a little responsibility on your shoulders. This is a chemical that shows up in everything from pharmaceuticals to flavorings—so sloppy storage could spell disaster, or at the very least, some ruined batches and anxious regulators. Here’s what matters: this isn’t just any old bottle you put on a shelf and forget. You have to treat it right, or pay the price later.
Every shop floor and lab manager knows there’s nothing magical going on here. Moisture, heat, sunlight—these are the same troublemakers that’ll mess up most chemicals. 2,3,5,6-Tetramethyl Pyrazine doesn’t explode at the drop of a hat, but things can go sideways if it gets too hot or damp. Humidity can clump up powders or corrode packaging. Direct sunlight can push some compounds to react or degrade over time. So, what’s the first rule? Grab a clean, sealed container and keep it in a cool, shaded spot. Top shelf, bottom bin, or fridge shelf, just make sure it’s away from light and heat sources.
Once you pop that seal, the clock starts ticking. Air exposure isn’t great for most compounds, and it leads to contamination, especially with the open containers common in labs that rush through protocols. I’ve seen good batches go bad in a matter of days because someone left a lid loose. So, take a minute, check if the cap is tight, maybe double up with a screw-cap seal or even wrap it in plastic film for an extra layer.
Stacking bottles in cramped shelves, all colors and shapes, can end badly. Some chemicals react to each other, and you really don’t want to learn that lesson after the fact. So, if there are acids, oxidizers, or flammable materials nearby, it’s time for a shuffle. Pyrazines don’t want neighbors that play rough. Keeping incompatible chemicals apart cuts risks, makes audits run smoother, and keeps you on the right side of safety regulations.
It’s a headache to hunt for a missing or mislabeled jar. Missteps happen fast in shared spaces: someone opens a container, grabs what they need, then puts it back on the wrong shelf. Next thing you know, there’s confusion or even accidents. Neat, clear labels and an updated logbook—that’s not bureaucracy, that’s covering your bases. A little discipline means no mystery jars at midnight, no scrambling through records when the auditor calls.
Handling 2,3,5,6-Tetramethyl Pyrazine doesn’t have to be dramatic, but accidents can get messy if you’re not prepped. Spills matter more than you’d think; even if you’re dealing with tiny amounts, ventilation, spill kits, and fire extinguishers need to be in reach. I’ve seen small mishaps turn long days worse simply because the right gear was shoved in the back closet. Setups that expect things to go wrong handle surprises without breaking stride.
Simple habits shape outcomes—storing chemicals like 2,3,5,6-Tetramethyl Pyrazine isn’t about memorizing regulations; it’s about protecting people, product, and reputation. The basics aren’t fancy, but ignoring them leads to real costs, both in lost material and unnecessary risks. By giving storage the attention it deserves, you build trust with everyone on your team and keep your operation running smoothly.
Anyone working in food science, pharmaceuticals, or chemical research has probably crossed paths with 2,3,5,6-Tetramethyl Pyrazine. Widely recognized for its nutty, cocoa-like aroma, this compound doesn’t just spice up flavor; it shows up in plenty of lab settings. Whenever a supplier offers a batch of this kind of chemical, someone almost always asks, “Can I see the certificate of analysis?” For folks outside the lab, it may sound like a minor request. But in my own experience handling bulk ingredients, that one document can make or break an entire project.
Years ago, I worked in a facility that blended specialty food ingredients. Twice, we dealt with suppliers that sent us what looked like top-shelf Tetramethyl Pyrazine—clean packaging, sealed drums, no off-odors. But opening the certificate of analysis, sometimes things didn’t add up. One batch had a purity six percent below what our formulation required. Another came with oversize particle specs that threatened to ruin our blending lines. It quickly became clear that you can’t lean on a handshake or fancy branding. The numbers and test results in that document serve as the real contract.
If quality parameters slip, it’s not just recipes that suffer. There’s the potential for regulatory trouble, wasted batches, or even safety recalls. It’s not just theory—one recall ties up weeks of warehouse space and has management breathing down everyone’s neck. What should have been a simple production run turns into a scramble, chasing emails and phone calls to pin down the source.
Reliable manufacturers share their certificate as soon as you ask. That includes data on purity, moisture content, melting point, and any contaminants. Labs usually want to see test method details too: what kind of chromatography, which standards, calibration methods. Some companies skip details or offer generic paperwork that fails to match the shipment. That’s a red flag. If a COA doesn’t name a specific lot number or match the drums delivered, I’ve learned to set that stock aside. I’ve lost some sleep deciding how to push vendors on authenticity, but it keeps the product line safe and builds real trust with customers.
A useful certificate cuts through marketing talk. Think clear numbers: purity in percent, moisture in milligrams per kilo, heavy metal limits right there on the page. In one project, I reviewed COAs from seven countries. The best ones had actual chromatograms attached and even the name of the chemist who signed off—no hiding. That openness let us trace a problem down the line within hours, not days.
People sometimes ask if demanding COAs adds cost or slows down orders. Strict sourcing brings its headaches, but it beats the risk of a rejected shipment. Fast-moving production runs rely on trust, but trust grows from clear data, not wishful thinking. Tough vetting up front saves trouble for R&D, saves operators from reruns, saves money for the whole team.
Asking for the certificate of analysis shouldn’t feel like negotiating a treaty. Producers should build sharing routines into every order. I’ve seen companies tie COA collection directly into their receiving protocols. Scanning paperwork—preferably digital, not faded photocopies—keeps everything aboveboard. Strong, open communication builds habits across the supply chain. Clear paperwork now means fewer headaches and more confidence in every kilo of Tetramethyl Pyrazine that hits the production floor.

| Names | |
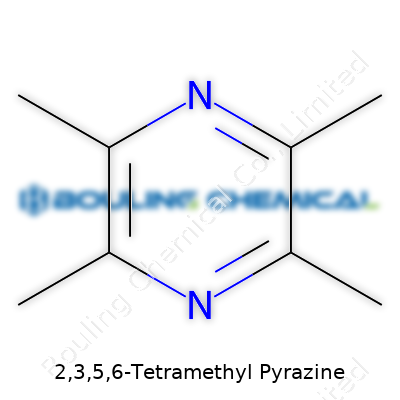
| Preferred IUPAC name | 2,3,5,6-Tetramethylpyrazine |
| Other names |
Tetramethylpyrazine Ligustrazine |
| Pronunciation | /ˌtuː ˌθriː, faɪv, sɪks ˌtɛtrəˈmɛθəl paɪˈreɪziːn/ |
| Identifiers | |
| CAS Number | 1124-11-4 |
| Beilstein Reference | 1718856 |
| ChEBI | CHEBI:9654 |
| ChEMBL | CHEMBL14237 |
| ChemSpider | 20568 |
| DrugBank | DB03915 |
| ECHA InfoCard | 18a9b23a-139b-4bc8-8f38-77fd6c49ca7a |
| EC Number | 208-349-1 |
| Gmelin Reference | 77859 |
| KEGG | C06135 |
| MeSH | D013718 |
| PubChem CID | 7018 |
| RTECS number | UJ3150000 |
| UNII | 5H31GI9502 |
| UN number | UN3166 |
| CompTox Dashboard (EPA) | DTXSID2050281 |
| Properties | |
| Chemical formula | C8H12N2 |
| Molar mass | Molar mass: 136.19 g/mol |
| Appearance | White crystalline powder |
| Odor | nutty; roasted; cocoa-like; earthy |
| Density | 0.958 g/cm3 |
| Solubility in water | Slightly soluble in water |
| log P | 0.91 |
| Vapor pressure | 0.0588 mmHg (25 °C) |
| Acidity (pKa) | 3.64 |
| Basicity (pKb) | 2.91 |
| Magnetic susceptibility (χ) | -84.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.5080 |
| Viscosity | 1.049 cP (25°C) |
| Dipole moment | 0.00 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 237.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -34.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3910.8 kJ/mol |
| Pharmacology | |
| ATC code | N06DX02 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07, GHS09 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Flash point | 61°C |
| Autoignition temperature | 514 °C |
| Explosive limits | Explosive limits: 1.4–8.1% |
| Lethal dose or concentration | LD50 oral rat 820 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 920 mg/kg |
| NIOSH | ST3995000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 2,3,5,6-Tetramethyl Pyrazine is not specifically established by OSHA. |
| REL (Recommended) | REL (Recommended): 1 mg/m³ |
| Related compounds | |
| Related compounds |
Pyrazine 2,3-Dimethylpyrazine 2,5-Dimethylpyrazine 2,6-Dimethylpyrazine Trimethylpyrazine |