2-(2-Piperazin-1-ylethoxy)ethanol didn’t pop up overnight. During the last half of the 20th century, as chemists deepened their interest in heterocyclic compounds, piperazine derivatives gathered growing attention. Research teams looked to tweak molecular frameworks for better results in pharmaceuticals and specialty chemicals, and the unique characteristics of 2-(2-piperazin-1-ylethoxy)ethanol caught their eye. Laboratories recognized its flexible backbone and basic nitrogen atoms, pushing it into pilot programs for advanced synthesis routes. From early patent filings in Europe and the United States to industrial process optimization reports, the compound’s story traces a path along scientific shifts—each generation building on the previous, shifting methods from small-flask batch work to scalable, cleaner manufacturing.
2-(2-Piperazin-1-ylethoxy)ethanol comes across not just as a chemical name, but as a promise for those looking to bridge water solubility with nitrogen functionality. With its unique blend of piperazine and ethanol groups, this molecule serves as a building block for all sorts of modern formulations, from active pharmaceutical ingredients to specialized corrosion inhibitors. While tucked away inside polymer or pharma plants, its clear, viscous appearance betrays nothing of its versatility. Colleagues often mention it in the same breath as niche intermediates that track big shifts in medicinal chemistry—a modest molecule, but an influential one in labs and industry workshops alike.
This compound usually appears as a colorless, oily liquid at room temperature. It carries a faint, amine-like odor that echoes its piperazine structure. Its boiling point sits comfortably above the usual solvents, hovering somewhere between 290–310°C, with a melting point well below water's freeze point. The density, generally measured close to 1.07 g/cm3, lines up with other oxygen- and nitrogen-rich organics. As for solubility, you’re looking at excellent water miscibility—ethanol’s polar influence prevailing over the ring. The piperazine's nitrogen atoms push basicity, so the molecule interacts easily with mineral acids and electrophiles.
Certified chemical suppliers assign strict guidelines for packaging and labeling. Purity levels usually reach at least 98%, with GC or HPLC quality checks on hand for confirmation. Product lots list water and chloride levels, sometimes also including heavy metal traces below 10 ppm. Standard labeling uses GHS pictograms, a UN number for safe transport, and custom batch ID tracking to trace every liter or drum. Documentation packs in shelf life, date of manufacture, and detailed hazard statements, reflecting strong regulatory pressure across global markets.
A classic synthetic route involves reacting piperazine with 2-chloroethanol under basic conditions. Alkoxide activation helps the ethoxy group displace halides efficiently. Some industrial systems employ two-phase solvent extraction, maximizing conversion by tweaking temperature, solvent, and reagent ratios. Continuous-flow setups and monitored reactors have made the process safer and more productive, pushing reaction yields higher and cutting down waste. Older lab notes stress slow addition and close pH monitoring to head off side reactions.
2-(2-Piperazin-1-ylethoxy)ethanol acts as a versatile building block. Chemists rely on both the hydroxyl and the secondary amine for further transformations. N-alkylation opens the path to more complex piperazine architectures. That lone primary alcohol handles esterification with organic or inorganic acids, creating surfactants or drug derivatives. Reaction with acyl halides gives specialty amides that slot into pharmaceutical scaffolds. Chemists often talk about ring substitution on the piperazine, letting medicinal ideas flow with greater flexibility. The compound handles reductive amination, ether formation, and nucleophilic aromatic substitution—skills that help it find a place in advanced R&D activities.
Trade names and synonyms fill technical catalogs for this compound. Common pseudonyms include N-(2-Hydroxyethoxy)piperazine, 2-(2-Piperazinylethoxy)ethanol, and sometimes HEPEP in research shorthand. Product codes differ across suppliers, with abbreviations slotted into internal inventory for pharmaceuticals, epoxy curing agents, and advanced coatings. International suppliers label it in several languages, depending on the target market. While beginners might tangle with the multi-syllabic name, seasoned chemists zero in on the alcohol and piperazine keywords, making them instantly recognizable.
Handling this molecule requires attention to detail. Safety data sheets stress eye and skin protection, with chemical goggles and nitrile gloves as standard. Inhalation exposure calls for fume hoods during transfer or mixing. Though acute toxicity stays low compared to aromatic amines, the compound causes mild irritation if handled carelessly. Storage tanks get fitted with corrosion-resistant linings, as the combination of ethanol and amine functionality will slowly eat away at exposed steel. Waste protocols demand containment and labeled drums, never down the drain. Operating manuals keep emergency sprinklers and eyewash stations handy, with spill cleanups focused on speedy neutralization and disposal.
Chemistry teams across several fields make use of this compound. Pharmaceutical development runs through piperazine intermediates at every stage, with this molecule acting as a launching pad for painkillers, antipsychotics, and enzyme inhibitors. In the materials world, coatings specialists experiment with it for curing, cross-linking, and boosting adhesion properties. Water treatment professionals add piperazine derivatives to their mix for selective ion removal and antiscalant applications. Specialty polymer brains fiddle with sterics and hydrophilicity, plugging the material’s alcohol group into custom polymer backbones, looking for that balance of toughness and ductility. Its surfactant skills also stretch into cosmetics, helping blend tricky formulations for creams and gels.
Laboratory notebooks show this compound at the heart of many new direction projects. Medicinal chemists chase selectivity by swapping alkyl chains and aromatic groups around the piperazine head. Polymers get more tailored thanks to the clear reactive handles on this molecule, making block copolymers easier to assemble with modern click chemistry. Biotechnology fields, keen on green synthesis, explore biocatalytic routes that use engineered enzymes to build the molecule from renewable feedstocks, not just petrochemicals. Instrument teams run advanced NMR, LC-MS, and IR methods to profile purity and trace byproducts, feeding lessons back into the process stream.
Toxicology teams measure acute and chronic effects in animal models, hoping to balance the compound’s reactivity with human safety. So far, no glaring system-wide toxicity jumps out at the concentrations used in basic research and specialty applications. Eyes and skin, though, will protest with redness and mild burns after direct contact. Long-term studies track organ impact at higher doses, with results pointing toward limited bioaccumulation. Routine audits make sure workplace exposure drops below the recommended milligram per cubic meter limits, and training materials highlight correct PPE and spill response procedures.
Interest in 2-(2-piperazin-1-ylethoxy)ethanol keeps rolling forward, largely because of the constant need for better, faster, and safer synthesis methods in both small-scale pharma labs and industrial plants. Pushes for more sustainable chemical processes hold promise for more efficient catalytic methods or biosyntheses. Teams exploring next-generation polymers need a small molecule that can slip into different architectures without demanding a host of new changes upstream. Emerging antimicrobial and adhesive formulations lean on piperazine derivatives, using their unique structure to cut through stubborn resistance. High-throughput screening and AI-driven molecule design pull more focus to this compound’s potential in areas just starting to take shape, pointing to a future built on careful chemistry, good science, and better global standards.
A lot of chemicals serve as the backbone for innovations in healthcare and industry. 2-(2-Piperazin-1-ylethoxy)ethanol is one of those backbone tools, coming out of chemical research labs and quietly shaping how modern medicine and chemistry function. Its reputation isn’t built on eye-catching headlines but on the small, technical leaps that add up.
This compound steps up in pharmaceutical research. Chemists use it as a handy building block to produce a range of medicines. Its piperazine ring gives flexibility in molecular design, allowing scientists to develop drugs that interact specifically with targets inside the body. For people living with conditions that require more effective drugs—think of patients struggling with complex mental health or neurological conditions—progress often links back to chemical innovations like this one.
2-(2-Piperazin-1-ylethoxy)ethanol also finds itself in medicinal chemistry as a linker or spacer while synthesizing molecules. The field moves fast, with a constant demand for new treatments, especially in cancer and infectious diseases. Patents list this compound in early-stage development of anti-tumor agents and CNS-active drugs. It sounds technical, but for researchers, it means they have one more card to play in the search for better therapies. People facing tough illnesses wind up benefiting, sometimes without ever hearing the names of the hidden chemical players involved.
Academic and industrial scientists often need reliable tools to join two different molecules together. 2-(2-Piperazin-1-ylethoxy)ethanol’s structure allows it to serve as a connecting piece or “spacer” within larger, more complex compounds. It supports the customization of drug molecules by creating the right shape or distance between essential parts.
Take peptide synthesis as one example. During the process, chemists add small chemical groups to control how peptides fold or behave. This compound helps tweak the behavior and solubility of molecules, improving how the final drug works in the human body. Fixing these problems early in the drug pipeline can make the difference between success and a treatment that never makes it past testing.
It’s not just drug discovery that benefits. Some specialty companies use 2-(2-Piperazin-1-ylethoxy)ethanol as a chemical intermediate in producing specialty polymers and surfactants. These materials pop up in coatings, industrial cleaners, and textile treatments. Quality control often means tracing ingredient performance back to chemicals like this one.
A chemical that shows promise on multiple fronts often raises safety and environmental questions. Handling chemicals with a piperazine core means thinking about exposure limits for workers and environmental impact. Mistakes and oversights in chemical handling put real people at risk in labs and production plants.
Practical solutions come out of real experiences. Companies keep people safe by combining proper ventilation, protective gear, regular monitoring, and effective waste management. Research should go hand in hand with regulatory transparency, so new uses and risks are updated and shared with everyone involved.
2-(2-Piperazin-1-ylethoxy)ethanol sits behind the scenes, enabling progress in pharmaceuticals and materials science. My time working on chemical research projects taught me to respect every step in the supply chain and appreciate the skill it takes to move an idea from a flask to a real product. Solutions start with knowledgeable people who treat these chemicals with equal parts caution and creativity.
Walking through a chemistry lab, I see nervous newcomers pause at the shelf with bottles like 2-(2-Piperazin-1-Ylethoxy)Ethanol. That long name spells a kind of seriousness. Anyone in research, pharmaceuticals, or chemical processing has run into specialty chemicals that demand respect. I’ve worked enough years in science environments to appreciate why safety isn’t just a set of printed rules. It comes from seeing real risks and real consequences turning into learning moments.
Chemical exposures sneak up on people. 2-(2-Piperazin-1-Ylethoxy)Ethanol has irritant properties, targeting the skin, eyes, and respiratory system. I always reach for nitrile gloves—latex breaks down with some solvents. Safety goggles do more than just check a box; I’ve seen a drip into an eye slow down work and send someone for medical evaluation. A lab coat isn’t a fashion statement—it’s saved more than one shirt and maybe a patch of skin from a spill.
Many labs have fume hoods, and there’s a reason—they move vapors out before they reach your nose or lungs. Years ago, an unmapped air current sent a whiff of something sharp from a benchtop flask straight to me, and I learned quick not to trust air flow. 2-(2-Piperazin-1-Ylethoxy)Ethanol can produce fumes, especially during transfers or reactions. Prolonged inhalation increases the risk for headaches, dizziness, or worse. Proper room ventilation goes far, but a fume hood adds a layer of certainty. Don’t lean over open bottles, and keep containers closed except during active use.
Storing chemicals may sound boring, but it saves headaches down the line. This compound fits best in a cool, dry spot away from direct sunlight. In my experience, clear labeling stops a lot of mix-ups. Sturdy, leak-proof bottles and segregation from incompatible substances (acids, oxidizers) keep small slip-ups from sparking bigger problems. I’ve seen water leak onto shelves causing unexpected reactions—precaution wins every time.
Every lab tells you to know the spill kit location, but not everyone practices using it. Quick response to a spill of 2-(2-Piperazin-1-Ylethoxy)Ethanol limits health risks and environmental mess. Absorbent materials, neutralizing agents, and proper disposal containers help. I try to keep spill cleanups smooth by running through dry runs in regular safety meetings. Sharing stories about mishaps helps the new folks take this step seriously.
No matter how careful you act, clean hands save the day. Chemicals cling to surfaces, and accidental face touches bring fast regret. I’ve seen busy researchers forget, only to get a nasty rash or eye sting. Frequent handwashing, changing gloves regularly, and not eating or drinking near chemicals stand out as commonsense habits. Old mentors used to tell me: “Don’t bring your work home with you,” meaning never take contamination outside the lab.
Every time a new chemical gets introduced, updating safety data sheets and training everyone makes a real difference. Regulators like OSHA provide standards, but the culture inside a lab drives real safety. Open discussion about health risks, proper use, and procedures also supports E-E-A-T standards, building trust and expertise throughout the team. Watching out for one another and holding each other to best practices creates an environment where both productivity and well-being can thrive.
Behind every complex chemical name waits a puzzle of atoms and bonds. The molecule in question, 2-(2-Piperazin-1-ylethoxy)ethanol, often finds its way into research labs exploring new drug candidates and materials science. This compound offers much more than just a jumble of fused rings and simple chains—it shows how functionality links with the core design of a molecule.
The chemical formula for 2-(2-Piperazin-1-ylethoxy)ethanol is C8H18N2O2. The backbone starts with a piperazine ring, which looks a bit like a six-sided stop sign if you sketch it out, with two nitrogens across from each other in the ring. From here, a two-carbon “ethoxy” arm extends, capped at the end by an ethanol group (an alkane chain with a terminal alcohol.) That might sound complicated, but try picturing the piperazine ring as the torso, the ethoxy arm as an outstretched arm, and the ethanol group as the hand. Nature loves to build bigger functions from smaller, repeating scaffolds.
From my time working with drug design software, I learned that the piperazine ring isn't just a static framework. It creates spots for other groups to connect, encouraging all kinds of reactions and changes in behavior. This adaptability supports medicinal chemistry. You see similar designs in drugs that touch everything from allergies to psychiatric disorders—think how antihistamines and antipsychotics frequently use piperazine-based scaffolds to join together different chemical features in one molecule.
In this case, the oxygen bridge—the ethoxy link—joins the piperazine to an ethanol group. The presence of the -OH (hydroxyl) group on the terminal end means this molecule dissolves well in water, setting it apart from more oily compounds. Better solubility improves how drugs behave in the body, since medications often need to move through watery environments. Working in the pharmaceutical industry, I often saw how these tweaks in structure could spell the difference between a promising lead and a wasted batch of test tubes. Even a small extension like an ethoxy bridge or a terminal alcohol can change biological compatibility and how the molecule acts in experiments.
Laboratories exploring new treatments often use derivatives of these piperazine compounds to create wide “libraries” of molecules for screening. Researchers choose these frameworks for their history of success, versatile reactions, and strong balance between flexibility and stability. Adding that ethanol end can help target specific enzymes or receptors more effectively. For example, a structure with increased water solubility might reach harder-to-access tissues if used in a drug trial. I’ve seen teams prioritize molecules like 2-(2-Piperazin-1-ylethoxy)ethanol exactly for this reason—hoping the right tweak opens up new doors in healthcare or diagnostics.
The uses for compounds with this structure reach beyond medicine. Industrial chemists sometimes adjust functional groups to develop better surfactants or materials with improved performance. With all these possibilities, safety stays crucial. Every new substituent, every shift in solubility, demands careful review. Early lab notebooks in my career showed how a single misjudged group could bring unwanted toxicity.
As more teams explore piperazine and ethanol-derivative combinations, the need for informed choices and rigorous data only grows. Deeper research—rooted in proven science—stimulates real breakthroughs, especially when clear reporting and collaborative learning lead the way.
Working in a chemistry lab brings both excitement and risk. Handling chemicals like 2-(2-Piperazin-1-Ylethoxy)Ethanol calls for respect and diligent habits. I remember my early days in the lab, tripping over poorly labeled bottles and stacks of mismatched containers. Even one careless misstep can spark bigger problems, from toxic fumes to chemical reactions that leave everyone scrambling. Protecting both people and property depends heavily on the storage strategies you build from the start.
Keep this compound in a cool, dry place. Warm temperatures increase vapor production and can nudge sensitive chemicals closer to their flash point. I’ve seen a hot summer afternoon in a poorly ventilated storeroom send the smell of solvents drifting down the halls—never a good sign. Air movement and temperature control stop those problems before they start. Consider storage away from direct sunlight, near an air vent, or in a climate-controlled cabinet. Refrigeration isn’t necessary here, but staying below 25°C makes sense, especially if humidity creeps up in your building.
Many labs lean on glass bottles for solvents and small-molecule reagents, though HDPE plastic can also work. Whatever you choose, it needs to seal tightly. Loose lids let vapor drift into the room, and moisture in the air can sneak in, setting off slow chemical changes you might not spot until later. Double-checking labels for both contents and dates prevents disaster down the line. I’ve taken over old storerooms filled with mystery bottles—trust me, you want every label intact and legible, or you end up guessing at shelf-stable safety.
This ethanol-based molecule should stay far from strong oxidizers and acids. Mixing the wrong bottles—deliberately or by accident—costs time, money, and safety. Chemists sometimes focus on their own projects and skip a review of the surrounding shelves, but it takes one bad spill to remind everyone that separation matters. Store 2-(2-Piperazin-1-Ylethoxy)Ethanol with similar, stable compounds, well away from anything marked as reactive or corrosive. Invest in spill trays, which contain accidental leaks and make cleanup less overwhelming—a tip I wish I’d known my first year in research.
No one should handle chemical storage without gloves, goggles, and a clear plan. Spills happen, even with the best intentions. Having dedicated spill kits and training everyone on small-scale cleanup keeps exposures to a minimum. Sharing simple best practices for both new interns and seasoned staff reinforces a culture where people look out for one another.
Modern labs rely on tight records—every chemical logged, checked, and sorted. Digital inventory systems cut down on expired or abandoned stock. Routine walk-throughs pick up problems that don’t show up in spreadsheets. I’ve solved more than one mystery leak or rogue odor by walking the aisles, finding a cracked lid or forgotten sample.
Safe storage habits come from the top down. When leaders and experienced staff put time into correct storage, no one sees it as optional housekeeping. Building this culture takes more than a poster on the wall; it means reinforcing, inspecting, and updating storage routines as new chemicals arrive. Small habits shape big safety outcomes.
I’ve always found that diving into the raw details of a chemical’s physical properties tells you a lot more than just what’s printed on a data sheet. The texture, color, and how the substance interacts with water or air matter for anyone planning to store, use, or handle 2-(2-Piperazin-1-Ylethoxy)ethanol in the real world. This compound, often called PPEE among chemists, doesn’t show up in everyday conversation. In labs and on factory floors, though, understanding its look, stability, and how it behaves with other materials helps avoid problems and manage risks.
In its pure, isolated form, 2-(2-Piperazin-1-Ylethoxy)ethanol tends to be a clear, colorless liquid. At times, slight yellow hues creep in if the sample’s been stored for a long stretch or subject to light. Most folks recognize its viscosity resembles that of common glycols. You get a material that pours smoothly and handles without stickiness, making it easier to mix than more resinous chemicals. Its texture feels almost slippery, which hints at its use in specialty solvents.
Solubility stands out as one of its main strengths. PPEE dissolves very well in water, thanks to the ethanol group in its structure. I’ve personally worked with blends where it mixed seamlessly, even at room temperature. There’s no visible separation or lumpy dissolving. That level of compatibility makes handling easier—spills don’t result in puddles of sticky residue, and cleanup goes quickly. Beyond water, it plays nicely with most polar organic solvents, including methanol and ethanol. This flexibility opens doors for broad use in pharmaceuticals or coatings, where mixing issues can stall production.
It takes real heat to push PPEE up to its boiling point. Under standard atmospheric pressure, expect it to boil above 200°C. That property gives it an edge in high-temperature processes. You’re not dealing with frequent vapor hazards at normal room conditions, which plays into worker safety and storage planning. The melting point sits far lower—so it stays liquid in most climates and rarely needs pre-heating. Reliable liquid state keeps dosing and dosing pumps running smoothly.
PPEE doesn’t evaporate quickly. That means workers or researchers don’t have to deal with quick losses from open containers. It shows good shelf stability when kept sealed and away from direct sunlight. Over the years, my experience has shown that it won’t cloud up or show major degradation unless you subject it to extreme acidic or basic conditions.
Though not classed with the most hazardous organics, PPEE calls for some care, especially if aerosols form during mixing or agitation. Eye and skin irritation turn up in the literature, which lines up with the piperazine group in its backbone. Regular gloves, goggles, and lab coats become non-negotiable. For storage, high-density polyethylene containers prove best—they don’t react and keep the compound stable for the long haul. Ventilation stops vapor concentration in space-constrained areas, but most applications don’t create much airborne material unless heated intentionally.
In my work, training makes the biggest difference. New staff pick up best practices by rehearsal—spills, splashes, even dosing errors happen less when top-line properties are known from memory. Posting easy-to-read summaries of boiling points, safe storage temps, and personal protective equipment in every area where PPEE makes an appearance builds collective competency. These steps sharpen safety and support efficient use in any operation that needs this versatile liquid.
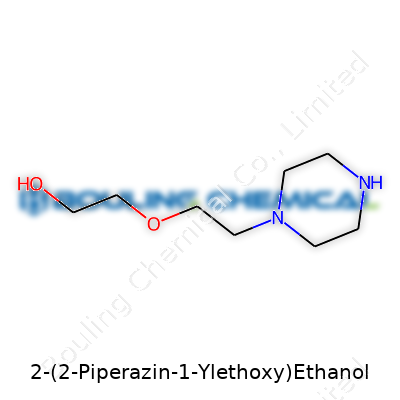
| Names | |
| Preferred IUPAC name | 2-[2-(Piperazin-1-yl)ethoxy]ethan-1-ol |
| Other names |
2-(2-Hydroxyethoxy)ethylpiperazine Bis(2-hydroxyethyl)piperazine 2-[2-(Piperazin-1-yl)ethoxy]ethanol 2-(2-Piperazinyl)ethoxyethanol |
| Pronunciation | /tuː-(tuː-paɪpəˈreɪzɪn-wʌn-ɪlˈɛθɒksi)ˈiːθənɒl/ |
| Identifiers | |
| CAS Number | 62426-11-5 |
| Beilstein Reference | 62686 |
| ChEBI | CHEBI:77963 |
| ChEMBL | CHEMBL131226 |
| ChemSpider | 33237546 |
| DrugBank | DB08317 |
| ECHA InfoCard | ## 13ea5f8c-4558-457a-8c99-6cb910ffe9e1 |
| EC Number | 629-21-4 |
| Gmelin Reference | 82232 |
| KEGG | C14323 |
| MeSH | D010944 |
| PubChem CID | 71336 |
| RTECS number | SQ2625000 |
| UNII | H4R2U7R58A |
| UN number | UN3439 |
| CompTox Dashboard (EPA) | DTXSID5059245 |
| Properties | |
| Chemical formula | C8H18N2O2 |
| Molar mass | 174.25 g/mol |
| Appearance | Colorless to light yellow liquid |
| Odor | Odorless |
| Density | 1.09 g/cm3 |
| Solubility in water | soluble |
| log P | -1.02 |
| Vapor pressure | 0.0000898 mmHg at 25°C |
| Acidity (pKa) | 8.9 |
| Basicity (pKb) | 5.35 |
| Refractive index (nD) | 1.520 |
| Viscosity | 23.7 mPa·s (25 °C) |
| Dipole moment | 3.06 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 128.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -307.6 kJ/mol |
| Pharmacology | |
| ATC code | N06AB05 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P264, P280, P302+P352, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | > 163.3 °C |
| Lethal dose or concentration | LD50 (Oral, Rat): 3,380 mg/kg |
| LD50 (median dose) | LD50 (median dose): 500 mg/kg (rat, oral) |
| NIOSH | GR3800000 |
| PEL (Permissible) | PEL: Not Established |
| REL (Recommended) | 10 mg |
| Related compounds | |
| Related compounds |
Piperazine N-Ethylpiperazine Bis(2-chloroethyl)ether 2-(2-Aminoethoxy)ethanol 2-(2-hydroxyethoxy)ethylamine |