My interest in the evolution of specialty chemicals goes back to years of tracking breakthroughs in organic synthesis. 2-(2-Chloroethyl)-1-Methylpiperidine traces its roots to mid-20th century labs where chemists, often working with limited analytical tools, needed better alkylating reagents and intermediates. As advances in heterocyclic synthesis gained ground in the 1960s and 1970s, researchers prioritized modifications of piperidine structures, seeking ways to expand pharmaceutical libraries and streamline agrochemical pipelines. Many innovations happened quietly; bench chemists in university and industry settings learned that introducing a chloroethyl group onto a methylpiperidine backbone could deliver unique reactivity. These early steps built the foundation for new alkylating agents, highlighting how historical momentum—more than dramatic inventions—shapes specialty chemical catalogs.
In practical use, 2-(2-Chloroethyl)-1-Methylpiperidine shows up as a purposeful building block. Its basic structure—a methylpiperidine ring bearing a 2-chloroethyl substituent—gives it a noticeable edge for those designing active pharmaceutical ingredients or fine chemicals. From firsthand experience in process chemistry, sourcing reliable intermediates like this often determines whether a project moves forward or bogs down in supply chain headaches. The product appeals most to sectors needing amine alkylation, selective modifications, or tailored side-chain insertion. I always look for suppliers who not only guarantee chemical purity on paper but also back claims with solid spectroscopic and chromatographic data.
Anyone handling this compound should expect it as a colorless to pale yellow liquid. You can tell a lot by the faint, sharp amine-like odor, which sometimes seeps out despite good seals. The density hovers around 1.02 g/cm³ at room temperature. It boils above 210°C, thanks to the chloroethyl group and ring structure. Being a secondary amine, it meets water with some reluctance, mixes easily with organic solvents, and keeps well under dry, dark storage. No one working with alkyl chlorides should underestimate the risk of hydrolysis, especially under acidic or basic conditions; even low-level moisture can chip away at its shelf life. Folks in my lab always keep it sealed and cold, away from light and other reactive chemicals.
Familiar labels read: “2-(2-Chloroethyl)-1-Methylpiperidine, >98% purity, CAS No. 1121-90-8.” Specs often include gas chromatography (GC) or liquid chromatography (LC) purity, water content by Karl Fischer titration, and residual solvents. Authentic suppliers provide batch-specific certificates of analysis, which include NMR and sometimes mass spec files for transparency. In the best labs, barcoded bottles help track usage and expiry. Safety data sheets must flag the alkyl chloride moiety: these materials need more attention than standard amine derivatives due to corrosivity and toxicity issues.
In synthetic routes, I’ve watched teams favor N-methylation of piperidine followed by chloroethylation at the 2-position. Typical steps involve methyl iodide or dimethyl sulfate to introduce the methyl group, then treat the intermediate with 1,2-dichloroethane in the presence of base. Chloride ion provides the leaving group for the final substitution. Good process chemists control temperature, dryness, and addition rates; skipping a step or shortcutting workup can introduce tough-to-remove side impurities. For scale-up, teams swap hazardous reagents for safer analogs where possible but find that faithfully following an optimized protocol produces fewer headaches—and a cleaner product.
This compound serves as a versatile actor in synthetic schemes. The 2-chloroethyl side chain acts as a classic alkylating agent, opening doors for nucleophilic substitutions with alcohols, amines, or thiol partners. I’ve seen clever modifications in medicinal chemistry, where researchers swapped the chloride for azide, then used click chemistry for late-stage diversification. Cross-coupling sometimes leverages the reactive chlorine, with palladium or nickel catalysts, to tie on aryl or vinyl groups. Acidic or basic hydrolysis gives the alcohol derivative, useful for further transformations. Its secondary amine can also engage in reductive amination or acylation, broadening its reach across combinatorial chemical libraries, especially for pharmaceutical screens.
Throughout supply chain offices, you bump into alternate names: N-Methyl-2-(2-chloroethyl)piperidine, 2-Chloroethyl-N-methylpiperidine, and 1-Methyl-2-(2-chloroethyl)piperidine. Multi-lingual catalogs sometimes simplify further, with trade names showing code numbers. It pays to double-check CAS numbers (1121-90-8) during procurement since similar-sounding compounds crop up in catalogs with confusing regularity, risking costly delivery errors or synthesis mishaps.
From my safety trainings, everyone handling this material should respect its hazards. The chloroethyl group poses risks, above all through skin contact, inhalation, or accidental ingestion. Alkyl chlorides like this can cause burns and sensitization; gloves, goggles, and lab coats protect skin while proper ventilation matters as vapor levels build up. Storage needs locked cabinets, dry inert atmospheres, and clear labels stating hazard categories. Every batch comes with a safety data sheet outlining toxicity, flammability, and disposal methods; these sheets give practical instructions, not just legal checkboxes. In spill scenarios, immediate containment, absorbent pads, and neutralizing agents stand ready, never far from a chemical fume hood.
Pharmaceutical R&D relies on this intermediate for tweaking lead compounds, especially where fine control of amine substitution patterns turns a mediocre molecule into a potent drug candidate. Crop protection labs use it to create new alkaloid analogs aimed at pest or weed management that skirts resistance trends. From hands-on work, I’ve found it especially reliable for testing new radiolabeled probes or as a starting point for CNS-active compounds. Polymer chemists, though less frequent users, explore its amine and alkyl chloride functional groups for engineering cross-links or branching in specialty resins that need both flexibility and reactivity.
University projects and private pharma start-ups keep the demand for this compound high. Ongoing work looks at new derivatives with improved blood-brain barrier penetration or targeted cytotoxic profiles. My conversations with medicinal chemists often highlight a relentless search for alternatives that offer selectivity without introducing excessive toxicity. Some labs work to simplify preparation, swap greener solvents, or cut residual impurities that could complicate regulatory approval. Automation and data-driven cheminformatics now allow faster iteration, where libraries draw on building blocks like 2-(2-Chloroethyl)-1-Methylpiperidine to diversify chemical space and accelerate early-stage drug discovery hits.
Toxicologists flagged this compound early as a cause for extra care. Animal studies indicate acute and sub-chronic exposure produces central nervous system and hepatic effects, typical for alkyl chlorides and substituted piperidines. In rodents, doses above threshold levels prompt ataxia, weight loss, and sometimes hepatocellular changes. Handling procedures must follow occupational guidelines to limit exposure well below reported LD50 values. In vitro assays demonstrate DNA alkylation, raising concerns about genotoxicity in high-exposure scenarios. Over years of reading safety literature, I’ve learned that toxicity often depends on route and duration of exposure, and chronic handling without proper PPE leads to cumulative risk. These realities push companies to strengthen training and implement exposure monitoring.
Interest in 2-(2-Chloroethyl)-1-Methylpiperidine continues to grow as demand for targeted amine derivatives spreads across the globe. Researchers flag this compound as a springboard for novel drug scaffolds, agrochemical leads, and new functional materials—especially as automated screening and molecular modeling guide the next generation of compound libraries. Synthetically, chemists pursue greener and safer preparation routes, favoring flow chemistry and high-throughput purification. Regulatory trends put a spotlight on sustainable disposal, driving suppliers to redesign supply chains for closed-loop processing or safer substitutes. Collaboration between academic and industrial labs opens fresh applications, from bioactive heterocycles to diagnostic agents, underlining the principle that strong foundational chemistry never goes out of style.
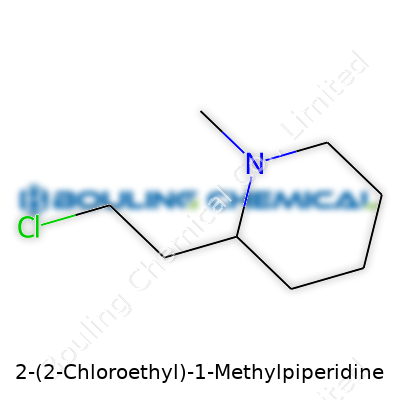
Chemistry gets interesting with structures like 2-(2-Chloroethyl)-1-Methylpiperidine. The name might give off a complicated vibe, but at its core, this compound pulls together a few recognizable pieces. At the center sits the piperidine ring, a six-membered, nitrogen-containing cycle. This ring remains a familiar backbone in everything from pharmaceuticals to industrial chemicals. The addition of a methyl group at the first nitrogen atom and a 2-chloroethyl chain at the second carbon turns this basic piperidine into a more complex and reactive molecule.
The arrangement of atoms shapes the compound’s characteristics. In this case, a nitrogen in the ring, a methyl group attached to it, and a chloroethyl chain dangling from the second carbon offer a variety of reactive sites. Chemists see opportunities in these spots for further modification, biological testing, or industrial reaction. Personally, when I first handled nitrogen heterocycles, the patterns of reactivity often surprised me. Even a “small” change, like swapping a potassium for a chlorine or adding a methyl group, tipped the scales in terms of solubility or behavior in a reaction flask.
What raises the stakes here is the chloroethyl side chain. Chlorine offers a leaving group, opening the door for substitution or elimination reactions. This feature signals potential for use in synthesis, especially in pharmaceutical labs where routes lead to more complex drug candidates. Anyone who’s tried to move a molecule around a multi-step pathway knows the value of that versatility.
A lone chlorine atom may not seem like much, but with synthetic chemistry, small additions can mean big risks. Chloroalkyl groups often bring toxicity concerns and sometimes even references to chemical weapon synthesis in regulatory texts. Chemists old and new owe it to themselves and the community to treat such structures with care. Years ago, I learned to check the Material Safety Data Sheets and regulatory status of every reagent before ordering, not just after. Knowledge protects not only the handler but also the environment around the lab.
These safety challenges create a responsibility for everyone involved—educators, researchers, suppliers. Training on chemical stewardship, not just the mechanics of synthesis, must become standard. Lax storage or disposal can lead to exposure hazards or regulatory trouble. Experience shows that the extra time spent on safety checks saves headaches and heartache later on.
With a structure like 2-(2-Chloroethyl)-1-Methylpiperidine, the focus should land on responsible use. It holds promise as a tool in synthesis, a potential intermediate in medicinal or agricultural chemistry, and even a subject for research into reactivity trends. Whenever a new synthetic pathway gets planned, considering downstream impacts—waste, toxicity, persistence in the environment—leads to smarter, safer science.
Access to up-to-date structural data, reaction pathways, and regulatory advisories could help demystify compounds like this and lower barriers to entry for new chemists. Accurate communication and clear facts lead to better outcomes across research and industry. By valuing both curiosity and caution, the field stays open to innovation while respecting safety and ethical standards.
2-(2-Chloroethyl)-1-Methylpiperidine doesn’t exactly make headlines, but within the walls of chemical plants and R&D labs, it’s a name folks don’t forget. Its value stands out most for chemists working on making other things. This compound, part of the piperidine family, helps stitch together more complex molecules. The nitrogen at its core can serve as an anchor point for different chemical reactions, and from what I’ve seen in lab syntheses, it often comes up when chemists need something with bite — a reagent that gets things moving.
In the pharmaceutical sector, the real action often starts with solving small puzzles, and 2-(2-Chloroethyl)-1-Methylpiperidine fits right into that role. The compound’s structure supports modifications important for active drug candidates. Medicinal chemists use it as a building block. In oncology research, the 2-chloroethyl group has been explored for its ability to disrupt DNA in cancer cells. Drugs known as alkylating agents, like cyclophosphamide, follow a similar structural logic. While 2-(2-Chloroethyl)-1-Methylpiperidine itself might not become a medicine you get at the pharmacy, it often plays a part in the journey toward one.
There’s another layer outside pharma — organic chemistry research. Libraries of small molecules, needed by teams exploring fuel additives, crop protection agents, or new plastics, benefit from versatile intermediates. Piperidine derivatives step in right there, where scientists try to weave together molecules with just the right properties. 2-(2-Chloroethyl)-1-Methylpiperidine brings both the flexibility of the piperidine ring and the reactivity of an alkyl chloride, which researchers can use to craft bigger, more complicated structures.
You can’t sidestep safety when dealing with any chloroethyl group. These groups can alkylate DNA and proteins — good in some cases, risky in most. If someone gets careless in handling this compound without gloves or proper ventilation, they’re inviting trouble. Training matters: I’ve watched seasoned chemists respect these compounds, knowing too many stories where lapses meant emergency showers or calls to doctors. Major producers put a lot of effort into safety data and labeling for this reason, but nothing replaces attentiveness on the ground.
Regulators keep a close eye on substances that have toxicity concerns. 2-(2-Chloroethyl)-1-Methylpiperidine falls into that group. Any waste from reactions using this compound carries risks, and disposal often involves tight controls to avoid environmental release. Process engineers now face the extra job of creating better handling protocols, using scrubbers or neutralizers in waste streams. We’re seeing more interest in greener chemistry, with replacements being developed that lower toxicity or reduce persistent residues. Progress in this direction relies both on new ideas in synthesis and on regulatory pushback.
Researchers keep hunting for better, safer ways to build the same structures that 2-(2-Chloroethyl)-1-Methylpiperidine helps create. Some turn toward outsourced contract research, or automation, hoping to keep direct human exposure low. More universities now teach green lab techniques early on. As knowledge grows and technology catches up, there’s hope for chems that do the job with less risk. In the meantime, the compound holds firm in its roles, especially wherever building sophisticated molecules makes all the difference.
2-(2-Chloroethyl)-1-Methylpiperidine, a compound with a mouthful of a name, carries a level of risk that shouldn’t be brushed off in lab or manufacturing settings. As someone who’s spent a good chunk of time around both bench work and scale-up, I’ve learned the hard way that chemicals with chloroethyl groups can cause serious harm—think severe irritation, possible toxicity, and long-term health consequences if things go sideways. The chlorine atom in particular raises the stakes, since related substances often show up on hazardous substances lists. Don’t let your guard down: accidents with these compounds rarely offer you a do-over.
Every time I’ve worked with reactive chemicals, proper personal protective equipment has been the major difference between just another routine job and an emergency room trip. Full nitrile gloves, a well-fitted lab coat, and chemical-resistant goggles are the bare minimum. Skin contact plays out way worse than a simple rash; absorption can trigger much deeper health issues. I learned to use a face shield for extra splash protection when pipetting or transferring any amount over a few milliliters—because nobody plans on accidents, but they always seem to show up when you get comfortable.
Fume hoods can feel like a luxury, until you’ve tried working without one and ended up with a pounding headache or worse. Even trace vapors from 2-(2-Chloroethyl)-1-Methylpiperidine can irritate your lungs and eyes, and long-term exposure brings serious respiratory trouble. In smaller college labs poorly equipped for strong airflow, I’ve had to cobble together extra fans and even ask for schedule changes to avoid rush hour occupancy. At industrial sites, make-up air and constant monitoring matter just as much as a single beefy exhaust fan.
This isn’t the kind of substance you toss into a random cabinet. I always use tightly sealed, clearly labeled glass containers—not all plastics can handle reactive chloro compounds safely. These go straight into a ventilated and designated chemical storage space, away from anything flammable, acidic, or prone to oxidation from light or heat. Environmental controls, like a temperature log and secure spill trays, have saved me from headaches more times than I can count.
Spills happen, even for the most careful of us. From my experience, thinking through each cleanup step before you ever open the bottle keeps disasters from spiraling. I keep absorbent pads, neutralizing agents, and a waste container close at hand—not buried in a distant storeroom. Working in a team, I made sure every member knew the emergency numbers, the eyewash ins and outs, and routes to the nearest exit. Running through a spill drill every quarter ended up more useful than any passive wall chart.
Complacency and overconfidence always invite disaster. I’ve watched experienced chemists skip the safety data sheet, only to run into trouble. Regular training sessions—with real scenarios and Q&A, not just silent powerpoints—build habits that stick. And when someone spots a safety shortcut or a potential improvement, that feedback deserves a serious hearing.
Working with substances like 2-(2-Chloroethyl)-1-Methylpiperidine requires more than checking boxes. Real safety means forming habits, sharing lessons, and encouraging vigilance. Keeping each other accountable builds trust and keeps everyone out of harm’s way. Investments in better equipment and routine practice pay off in peace of mind—far more valuable than hoping luck holds out one more day.
2-(2-Chloroethyl)-1-methylpiperidine walks into the lab with a reputation. It’s reactive, carries potential toxicity, and exposure brings real hazards to skin, eyes, and lungs. These traits can’t be shrugged off, so storing this compound takes a practical and deliberate approach. Reading the safety data sheet, I’ve learned that proper storage can make the difference between routine lab work and a costly emergency.
Forget fancy storage trends. This substance asks for what many dangerous organics demand: a tightly sealed glass or high-density polyethylene container. Tossing it in a random cabinet or metal can invites trouble—corrosive vapors and chemical incompatibility are the faster routes to incident reports. A well-sealed container stops any vapor from slipping out and contaminating the air.
The ideal storage site? Find a cool, dry spot, away from direct sunlight and heat sources. Warmth and UV light nudge decomposition, which doesn’t just impact the compound’s reliability, but builds risk inside the container. At my last research post, we’d use glove boxes or dedicated flammable material refrigerators. Never try freezing this material unless a chemist who knows the nuances of its solubility and physical states could vouch for it.
In my own work, a sloppy lid or overloaded shelf became the root cause of several safety rounds with the EHS team. Too many people assume O-ring gaskets all work the same, but solvent fumes will eat a regular rubber seal alive by the end of the week. Viton or Teflon caps stopped those worries cold. And always label every flask, bottle, and vial with plain language and date of receipt. Guesswork in a chemical storage room breeds confusion, and confusion breeds accidents.
Any chemical with reactive or corrosive vapor potential deserves storage in a vented chemical cabinet with good airflow to the outside. Fume hoods stay reserved for daily use. I watched a colleague forget this principle, inadvertently leaving a volatile piperidine in a metal cabinet—eventually, corrosion began to compromise the shelving, and a cleanup cost half a day and more than a little embarrassment.
Neighbors make all the difference. Pairing 2-(2-Chloroethyl)-1-methylpiperidine next to oxidizers, acids, or bases risks violence—think fire or explosion. Separate incompatible materials onto different shelves or, for higher-risk chemicals, into different rooms.
Following local regulations and OSHA guidelines matters—these rules didn’t show up to make life tough for researchers. Instead, they shape habits proven to cut down on exposure and accident rates. Capping quantities also helps: store only what’s needed, and rotate stocks to avoid aging containers.
Stocking spill kits—neutralizing agents, absorbent pads, goggles, gloves—is non-negotiable. Training every hand in the lab to use these tools, and holding drills that mimic the worst-case scenario, brings home the seriousness of the storage game.
A solid approach boils down to: stick to stable containers, keep compounds cool and dry, never slack on labeling, segregate hazards, and respect the tight safety culture developed over years of experience. Chemicals like this one don’t forgive lapses. Every ounce of preparation pays back in calm, predictable days at the bench.
Anyone who spends time around chemical research or industrial labs starts to notice the unique importance of identifiers like CAS numbers. For 2-(2-Chloroethyl)-1-Methylpiperidine, the CAS number is 34998-93-5. Its molecular formula is C8H16ClN. Sitting at the intersection of organic chemistry and industrial application, these identifiers turn confusion into clarity. I remember a colleague once ordered a compound for an experiment only to realize wrong chemical came because the supplier worked with a slightly different name for the same structure. Precision here saves both time and safety.
CAS numbers didn’t become industry standard out of thin air. The discipline of chemistry carries a bit of chaos with similar names, isomers, and lengthy descriptions. Rather than getting lost in lengthy chemical names, a CAS number cuts straight through ambiguity. Government agencies, universities, and factories work with these tags because nobody wants a costly or dangerous mix-up. In the world of 2-(2-Chloroethyl)-1-Methylpiperidine, a proper CAS number makes procurement, handling, and regulatory compliance far more reliable.
This compound, with its piperidine backbone and a chloroethyl group, lands squarely in specialty chemical circles. Its formula, C8H16ClN, tells you a fair bit: the single chlorine atom signals some degree of reactivity, often drawing attention from researchers working with alkylating agents. The addition of a methyl group to the piperidine ring changes the compound’s properties, giving it a distinct set of reactivity and solubility patterns compared to simpler analogs.
Once a compound includes a chloroethyl group, accident prevention moves to the front of the line. Chemical safety records show that mishandling this family of compounds can lead to health concerns, such as irritation or, in worse cases, impacts on the nervous system. On-site chemists always insist on protective gear and proper fume hoods. In past projects, lab safety audits repeatedly circled around making sure containers held what they claimed, with the correct CAS number to back up the labeling protocol. It’s not just academic; mistakes end up on the front page.
Despite digital inventory tools, some companies still lean on handwritten labels. Mix-ups lead to hazardous conditions and legal headaches. The chemical world doesn’t need big investments in order to get compliance right—just some discipline in labeling and digital cataloging. I’ve helped teams set up inventory apps that link CAS numbers straight to SDS (Safety Data Sheet) databases, which means everyone from students to senior researchers keeps records airtight. It’s less about spending big and more about building a culture of double-checking facts.
Behind every bottle with a CAS number and true molecular formula stands a community of scientists doing their best to keep each other safe and informed. For 2-(2-Chloroethyl)-1-Methylpiperidine, listing out “34998-93-5” and “C8H16ClN” on every shipment, order, and experiment log helps avoid the kind of problems nobody wants to see. With solid habits, accurate documentation, and staff education, both routine research and industrial projects get a little less risky and a lot more trustworthy.
| Names | |
| Preferred IUPAC name | 1-methyl-2-(2-chloroethyl)piperidine |
| Other names |
1-Methyl-2-(2-chloroethyl)piperidine NSC 21907 |
| Pronunciation | /tuː tuː ˈklɔːrəʊˌɛθɪl waɪ ˈmɛθɪl paɪˈpɛrɪdiːn/ |
| Identifiers | |
| CAS Number | 33705-84-9 |
| Beilstein Reference | 1206810 |
| ChEBI | CHEBI:196087 |
| ChEMBL | CHEMBL2104127 |
| ChemSpider | 159181 |
| DrugBank | DB08308 |
| ECHA InfoCard | 03d106eb-ea57-4380-b442-184005d8e55f |
| Gmelin Reference | Gmelin 84159 |
| KEGG | C19675 |
| MeSH | D014237 |
| PubChem CID | 10227595 |
| RTECS number | TD1750000 |
| UNII | 67Z84N5718 |
| UN number | UN2733 |
| CompTox Dashboard (EPA) | DTXSID6020445 |
| Properties | |
| Chemical formula | C8H16ClN |
| Molar mass | 161.66 g/mol |
| Appearance | Colorless liquid |
| Odor | amine-like |
| Density | 0.988 g/cm3 |
| Solubility in water | Slightly soluble |
| log P | 1.9 |
| Vapor pressure | 0.33 mmHg (at 25°C) |
| Acidity (pKa) | pKa = 11.2 |
| Basicity (pKb) | 5.29 |
| Magnetic susceptibility (χ) | -7.23 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.498 |
| Viscosity | 0.92 cP |
| Dipole moment | 3.15 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 362.6 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | L01AB03 |
| Hazards | |
| GHS labelling | GHS02, GHS06, GHS07 |
| Pictograms | GHS06 |
| Signal word | Danger |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P210, P264, P280, P301+P312, P305+P351+P338, P337+P313, P330, P501 |
| NFPA 704 (fire diamond) | 1-3-2 |
| Flash point | 79°C |
| Autoignition temperature | 225 °C |
| Explosive limits | Lower: 1.5% Upper: 9.5% |
| Lethal dose or concentration | LD50 oral rat 282 mg/kg |
| LD50 (median dose) | LD50 (median dose): 88 mg/kg (oral, mouse) |
| NIOSH | SKC20740 |
| PEL (Permissible) | PEL (Permissible) for 2-(2-Chloroethyl)-1-Methylpiperidine: Not established |
| REL (Recommended) | No REL established |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
1-Methylpiperidine 2-Chloroethylamine 2-(2-Chloroethyl)piperidine 2-(2-Bromoethyl)-1-methylpiperidine 2-(2-Chloroethyl)-1-ethylpiperidine |