2,2,6,6-Tetramethylpiperidine found its way into chemical literature before most of us picked up our high school textbooks. Chemists hunted for stable amine bases, trying to tune reactivity through structure in the 1950s and 60s. Bulky amines like this one became handy tools, guiding research toward steric hindrance as a design element. Over decades, this molecule cropped up in discussions about everything from organic synthesis to polymer stabilization. Its popularity in the lab owes a lot to the search for non-nucleophilic bases that don’t meddle with sensitive reactions. Looking back, that key insight—building in bulkiness—steered a whole generation of organic chemistry.
Ask around in any lab that deals in fine chemicals and the name “2,2,6,6-Tetramethylpiperidine” often gets shortened. Some call it TMP; others stick to its full form for paperwork. What matters more is its presence in small bottles on many shelves. Researchers use this compound for synthesis, especially because of its rigid and crowded structure. It feels a bit niche—no one picks it up for general solvent cleanup or routine titrations. Instead, its applications emerge where selectivity, low nucleophilicity, and consistent performance count. The unique architecture keeps it in demand among chemists who want clean, predictable results.
Pure 2,2,6,6-Tetramethylpiperidine looks like a colorless oil with a sharp, distinct odor—something between amines and faint medicinal notes. Boiling point sits around 155-156°C at standard pressure, making it volatile enough to handle with care but not so tricky as to require major lab modifications. It mixes comfortably with organic solvents such as ether, benzene, and hexane, but keeps away from polar protic liquids. The molecule’s structure, dominated by four methyl groups on a six-membered ring, casts a large shadow sterically while still letting that nitrogen atom do its job. It doesn’t leak electrons easily, so you don’t see radical reactions here. The bulk insulates the key parts of the molecule, pushing it toward selective reactivity and away from unwanted side reactions.
Manufacturers package TMP in amber glass, with purity usually above 98%. Labels flag hazards—irritant, flammable liquid, avoid skin or eye contact, keep tightly sealed. CAS number 768-66-1 shows up on every document, alongside standard hazard pictograms and phrases in plain type. Every shipment brings the expected material safety data sheet, spelling out everything from storage temperature to disposal methods. Big players in the chemical supply chain stock it as both analytical and industrial-grade, and most catalogs include shelf-life information. Batch-specific data means you can track back from any experiment to the original bottle, which matters for both reproducibility and safety investigations.
TMP doesn’t simply pop out of thin air. Industrial chemists often prepare it by alkylating piperidine with methylating agents under carefully controlled conditions. The choice of protecting groups, reaction temperatures, and purification steps determines overall yield and purity. Lab-scale synthesis calls for patience: controlling reaction time, maintaining inert atmosphere, and careful distillation at reduced pressure. Waste streams carry the marks of strong bases and methyl halides, reminding anyone working in the area to monitor both fumes and temperature at all times. Getting this compound pure enough for research use sometimes takes several rounds of vacuum distillation or recrystallization.
Use TMP in the lab and a few common patterns show up. Its main attraction is as a strong, non-nucleophilic base—a tool to pull protons without attacking sensitive functional groups in organic synthesis. You see it in enolate formations, deprotonation steps for carbonyl compounds, and as a backbone for generating other ligands or catalysts. It stands up to strong electrophiles but rarely forms undesired side products. Functionalizing TMP itself sometimes means swapping out hydrogen atoms adjacent to the nitrogen, with chemists developing derivatives for new ligands or sterically demanding additives in transition metal catalysis. Comparing it to more basic or less crowded amines, TMP consistently stands out for selective and predictable performance even in complex reaction mixtures.
Nobody wants to write out “2,2,6,6-Tetramethylpiperidine” on every form, so chemists quickly shorten it. TMP jumps off labels, but catalog entries may use 2,2,6,6-Tetramethylhexahydropyridine, Quadramine, or the registry number instead. Look at international suppliers and the translations keep multiplying—German, Japanese, and Russian catalogs each have their preferred local versions. The range of names means that double-checking the structure or registry number gets a chemist out of mixups. Over time, though, “TMP” built a reputation strong enough that misunderstandings rarely slow down an order or a project.
Safety conversations about TMP never feel boring, especially if you remember your own glove tearing during a cleanup. Unpleasant fumes, skin irritation, and flammability anchor the list of problems. Gloves, goggles, and a decent fume hood rank as absolute essentials. Spills don’t dissolve quietly; instead, you get a stubborn film that refuses to vanish without proper solvents. Waste disposal runs through organic bins, never the drain, and storage away from heat or ignition sources needs constant vigilance. Labs often limit bottle sizes to a few hundred grams for accident prevention, while larger operations lock up bulk containers behind access controls. Every safety officer I’ve met insists on annual training for anyone using TMP in routine synthesis.
TMP belongs to a narrow circle of reagents that shape both academic research and high-value manufacturing. Fine chemical synthesis, particularly where selectivity steers profitability, features it heavily. Medicinal chemistry groups grab it for stepwise functionalization in drug development. Material scientists lean on its stability for preparing specialty polymers and as an additive to protect susceptible materials from radical degradation. Asymmetric synthesis strategies sometimes exploit TMP or its derivatives to produce chiral ligands, tuning catalyst performance for enantioselective processes. Battery researchers study TMP-based derivatives as part of the search for stable, high-voltage organic electrolytes.
Ongoing research keeps turning up new ways to use TMP, which speaks to the adaptability hidden in its compact structure. Recent years have seen scientists focus on structural modifications—adding functional groups, linking with metals, or building more complex scaffolds. Some of the most intriguing projects tackle environmental impacts, aiming for greener production pathways with improved atom economy and reduced waste. Peptide synthesis, transition metal chemistry, and even supramolecular assembly projects report steady benefits from customized TMP analogs. Whenever a synthetic route hits a snag at the deprotonation step, someone in the group usually suggests bringing TMP into the mix, betting on its reliability.
No one lasts long in the field without paying attention to potential hazards. Toxicology studies turned up moderate acute toxicity for TMP, with main concerns pegged to eye, skin, and respiratory irritation. Chronic exposure data still lag behind other industrial amines, which nudges safety officers to err on the side of caution. Animal studies show reversible effects at low doses, but higher exposures drive nervous system and respiratory problems. Environmental scientists keep an eye on its persistence in water and soil, but so far major contamination events don’t often make headlines. Rigorous safety guidelines, especially for industrial operators and frequent users, rank as policy priorities—engineering controls, personal protective equipment, and clear workplace education all carry more weight than ever.
TMP’s future hangs on chemistry’s growing hunger for precision and predictability. Applications in green and sustainable synthesis look promising, especially as labs adopt stricter rules on hazardous byproducts and atom efficiency. Demand grows in transition metal catalysis and materials science, where new ligands with controlled steric footprints are in short supply. Efforts to reduce both volatility and environmental persistence may drive molecular tweaks—phosphonate, carbamate, or sulfonate substitutions promise to expand its use further. Automated synthesis and flow chemistry add another angle, since TMP’s clean reactivity lines up with the exacting demands of instrument-driven production. Even with old friends like TMP, the ever-changing landscape keeps pushing chemists to experiment more, dig deeper, and look beyond the standard reaction playbook.
At first glance, the name 2,2,6,6-Tetramethylpiperidine can feel like a tongue twister only a chemist would love. Get past the jargon and it reveals itself as a surprisingly important compound for anyone working in laboratories or manufacturing. The main reason people reach for this molecule is its reputation as a strong, non-nucleophilic base. In plain terms, that means it reliably grabs protons in chemical reactions without jumping in and starting side reactions. That alone puts it near the top of the list for safety and control in organic synthesis.
Back in undergraduate chemistry labs, finding a strong base that didn’t mess around with unwanted byproducts was always tricky. Sodium hydroxide, for example, may get the job done, but often leaves a mess to clean up in complex reactions because of its tendency to poke its nose where it doesn’t belong. 2,2,6,6-Tetramethylpiperidine keeps its head down and lets the real transformation happen, making it invaluable in steps such as deprotonation or the formation of enolates.
Organic synthesis forms the backbone of pharmaceuticals, materials science, and even flavors and fragrances. Imagine trying to create a precise drug molecule for cancer research. Side reactions eat up time and money and can derail months of effort. A dependable base lets chemists skip the headaches and focus on designing better molecules instead of cleaning up sloppy chemistry. That’s something I’ve seen first-hand working with teams trying to make progress on stricter timelines and tighter budgets.
While most people will never see a bottle labeled “2,2,6,6-Tetramethylpiperidine” outside a research setting, its presence powers industries in more ways than most realize. The compound steps into the spotlight in big manufacturing runs of specialty polymers and fine chemicals. Companies prize it for the precision it brings to multi-step processes. Since quality control and regulatory demands grow stricter every year, tools that remove reaction guesswork become even more valuable.
Some may wonder if its high cost or specialized use limits its reach. Truth is, chemistry pays for reliability. When the cost of a single bad batch can run into thousands—or much worse with regulated pharmaceuticals—the right chemical earns its keep many times over.
No story about lab staples would be complete without mentioning safety. Like many organic bases, this compound brings health risks. It’s toxic if mishandled and can irritate eyes or skin. Labs need solid training and ventilation to handle it responsibly. I’ve sat through enough safety meetings to know that one careless move with reagents like this means not just ruined work, but sometimes a trip to the ER.
Looking ahead, manufacturers can ease the environmental toll by recovering and recycling spent material where possible. Chemists also explore alternative, greener non-nucleophilic bases for less hazardous waste. Still, for now, 2,2,6,6-Tetramethylpiperidine keeps its spot in the chemist’s toolbox, making a lot of modern chemistry possible.
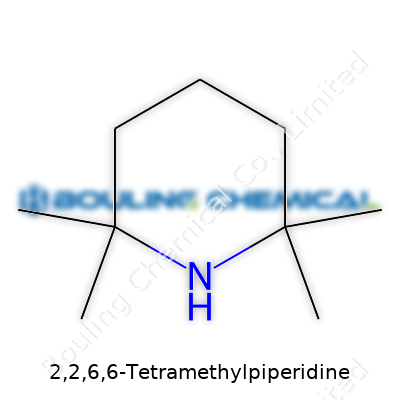
Sometimes, a long chemical name offers hints about its skeleton. Take 2,2,6,6-Tetramethylpiperidine. The name looks intimidating, but after taking a closer look, it’s quite a practical molecule. Its formula is C9H19N. The “piperidine” part means the molecule centers around a six-membered ring holding five carbon atoms and one nitrogen atom. The “2,2,6,6-tetramethyl” part means that each of the carbons at positions 2 and 6 carry two methyl groups. Draw it out, and you’ll spot the piperidine ring with four methyl group flags sticking out—two near the nitrogen, two across the ring.
Chemists often sketch its structure to emphasize the methyl-Avalanche at those two carbons. The nitrogen sticks out, surrounded by a crowd of methyl groups. I’ve seen this molecule pop up across textbooks and research projects, the bold methyl clusters hard to forget.
This compound gets more mileage in labs than most people realize. Its bulging shape, stiffened by its methyl groups, transforms its behavior. In chemical reactions, bulk matters. Here, that extra girth around the nitrogen stops a lot of side reactions from taking off. That makes 2,2,6,6-tetramethylpiperidine handy as a base—a tool I’ve reached for in organic synthesis because it can grab protons without causing extra trouble elsewhere.
This cage of methyl groups blocks the nitrogen from attacking nearby molecules. In some reactions, that's the difference between a clean result and a mess of side products. Picking the right base often means the difference between a late night cleaning up a column or a free evening.
Chemists used to lean on bases like triethylamine, but those don’t always avoid unwanted tangles. Here, tetramethylpiperidine shines, especially in sensitive reactions, such as working with strong electrophiles or forming certain bonds. Its practical value builds on this tailored shape.
In industry and research, reliable reagents keep costs and headaches down. Tetramethylpiperidine doesn’t just stay useful as a base. It’s a key ingredient when making stable nitroxyl radicals, like TEMPO, which I’ve learned are crucial for oxidation reactions and even some “green chemistry” processes. That means less waste, which everyone can get behind.
But every useful chemical has a flipside—safety. Lab accidents don’t make headlines, but they ruin days. Tetramethylpiperidine gives off a strong odor, and it can irritate eyes and skin. I keep the gloves nearby when working with it, no matter how innocuous reviews sound. And labs using large quantities need proper ventilation: headaches or worse follow when safety steps get skipped. Safety data never feels unnecessary with amines, even ones that look tame.
With compounds like tetramethylpiperidine, the story isn’t just about structure. Adopting safer handling practices—using fume hoods, keeping accurate labels—protects everyone. Integrating greener alternatives or even designing cleaner derivatives might go another step, reducing waste and exposure. Chemists look at what works in the lab today, but I like to think future students will work with even safer, more sustainable versions. The ideas and applications keep evolving.
So, familiar names like 2,2,6,6-tetramethylpiperidine hide stories of clever design, daily decisions, and a push toward safer, more sustainable science. Each bottle holds more than molecules—there’s a piece of chemical history and hope for better solutions down the line.
Pulling out a bottle labelled “2,2,6,6-Tetramethylpiperidine” always makes me check the Safety Data Sheet twice. Plenty of chemicals get tossed around labs with little fanfare, but this one draws attention for good reason. It’s got a strong ammonia-like stink, and after years spent working with organic compounds, you start to treat anything that sends vapors across a room with a few extra steps of respect.
This compound stands out as an efficient base and radical scavenger. High reactivity often walks hand-in-hand with toxicity. 2,2,6,6-Tetramethylpiperidine irritates skin, eyes, the respiratory tract—it’s one of those chemicals that leaves you coughing if a whiff escapes the bottle. Studies show if you splash or inhale enough of it, you risk serious tissue damage. Some folks shrug it off, saying, “Everything’s dangerous at the wrong dose.” Yet, few would ignore how fast irritation sets in with this stuff.
I recall a grad student splashing her glove with a solution containing it. She felt the burn through nitrile. The fix? Peel the glove, flood her hand with water, seek help. Hazards aren’t theoretical—one slip, and your day spins sideways.
The Material Safety Data Sheet tells you to avoid skin contact, wear goggles and gloves, and keep everything well ventilated. That’s basic protocol for plenty of solvents and reagents, but skipping a fume hood, or forgoing full-face protection with 2,2,6,6-Tetramethylpiperidine, sets you up for trouble. Standard-issue respiratory masks rarely block vapors. The law might not force fume hoods for every use, but plugging in a fan and opening a window won’t cut it. If this compound gets airborne, the sting in your nose makes it unmistakable.
Some labs keep extra supplies of calcium gluconate gel nearby—hospitals rub it on skin exposed to certain alkylating agents. While that doesn’t always help with piperidines, having a robust first aid kit on hand never hurts. It’s basic lab culture, built from years of watching folks overestimate how much PPE protects them.
Improving safety with 2,2,6,6-Tetramethylpiperidine starts with genuine training, not just a stack of signed forms or an annual PowerPoint. New researchers should watch senior folks set up reactions and clean up spills. Peer stories stick better than warnings taped to the wall. Regular drills and refreshers on handling spills help too; confidence only grows with practice.
Switching to digital logbooks and safety checklists nudges everyone to stay on task. Nobody likes filling in paperwork, but typing “checked fume hood” or “confirmed gloves/goggles before use” turns safety steps into habits. Giving people the chance to call out unsafe behavior without punishment also sets a standard.
Looking for alternatives never hurts, either. Ask if a milder base works for the project, or if safer conditions make sense. Chemistry keeps moving, so safer substitutions for legacy reagents come up now and then. No research award deserves a trip to the ER.
2,2,6,6-Tetramethylpiperidine speeds up chemistry, but nobody wins if it cuts corners on safety. Old scars and close calls push labs to rethink their routines. People matter more than any reaction yield—handle risks with open eyes, real talk, and a readiness to adapt.
Any chemist who spends time around research labs knows chemicals don’t forgive sloppy habits. 2,2,6,6-Tetramethylpiperidine stands as no exception. You’ll spot it on shelves where anyone working with specialty organic compounds stores their tools. Controlling the way it's stored isn’t about bureaucracy—it comes down to user safety, longer shelf life, and avoiding unnecessary waste.
If you pop the cap on a bottle of this stuff, you’ll notice an odor right off the bat. That's a signal that we’re not dealing with something you’d want loose in the air. Exposure brings a risk of irritation—eyes, nose, and skin don’t appreciate much contact.
Keep it far from kids, snacks, and break rooms. Regular shelving in a ventilated, low-traffic spot may work fine in an academic lab, but few things ruin a day quicker than a spill or unwanted mixing with incompatible substances.
I keep mine in well-sealed containers made from glass or high-grade plastic—never metal, since certain metals don’t get along with organic amines. Tightly closed, upright bottles help prevent leaks. Moisture in a storage area spells trouble, as it can prompt decomposition or set off side reactions. Some folks use desiccators or place a simple silica packet with the container. That buys extra peace of mind if your lab’s humidity creeps up.
Storing at room temperature, away from direct sunlight, works in most cases. Placing the bottles behind locked cabinets controls both safety and access. If you can store flammable solvents, you’ll have a sense of the right level of caution.
With gloves on and goggles fitted tight, I use low-splash transfer techniques. Pouring into a clean, dry beaker, I take care not to let air flow whip up fumes. Some labs will set up downdraft hoods just for these processes. If a spill happens, plenty of absorbent, lots of ventilation, and prompt disposal keep folks safe and avoid larger headaches.
Don’t return leftovers to the main supply; contamination stories spread fast, and no one wants to pay for wasted batches. Any rags or tissues contaminated go in a clearly marked, chemical waste bin. If your team is new, a five-minute review goes a long way in reducing accidents.
Looking at accident reports and my own experience, accidents seldom come from the chemical itself—they start when people ignore small steps. Training new students before they use the compound, labeling shelves and containers in plain language, and scheduling monthly checks make the difference.
For unexpected exposures, having a stocked eyewash and safety shower in the room is non-negotiable. Poison control contact info taped near the bench may seem over-cautious, until the day it saves hours of worry.
If storage areas lack climate control or face frequent temperature swings, I’ve found insulated containers or simple weatherproof cabinets keep supplies stable all year. Don’t cut corners to save on electricity or shelf space—spending a few dollars on better containment beats replacing lost material or cleaning up after a spill.
Chemical safety is only as good as your least careful habit. Storing and using 2,2,6,6-tetramethylpiperidine responsibly means looking out for yourself and the people who share the space. Every good day in the lab starts long before the flask hits the hot plate—it begins in the way you treat your materials from the moment they arrive.
Step foot in a chemical lab, and sooner or later you’ll spot a bottle of 2,2,6,6-Tetramethylpiperidine (TMP). No fancy nameplates or glitzy marketing, just a practical tool scientists and engineers reach for during tough problems. The bulky shape of this molecule makes it a standout in the world of organic chemistry, and folks outside the industry rarely hear its name. Still, TMP plays a bigger role than most imagine, and the work it makes possible reaches farther than the nearest beaker or test tube.
Most days, TMP acts as a strong, non-nucleophilic base—a kind of helper that grabs protons, setting the stage for more complicated molecules to grow. Medicinal chemists often rely on TMP during the early steps of synthesizing drug candidates, because it doesn’t interfere with the rest of the recipe. Drugs for everything from heartburn to cancer start with reactions no one could finish without reliable bases. TMP makes the difference between a promising idea and an actual pill on the market.
Pharmaceutical scale-up isn’t where the story ends, though. Manufacturers of specialty chemicals and agrochemicals chase unique reactions that old-fashioned bases can’t manage. TMP keeps the side products low and the main product high, which means fewer headaches and less waste. In an era of rising chemical regulations and tight margins, cutting down on byproducts translates into real money saved—and less environmental frustration.
Walk into any electronics store and pass rows of devices made with high-performance plastics. The people designing these gadgets count on reliable compounds to control the polymerization process. Here, TMP helps as a catalyst component, shaping the molecular weight and structure of products like polyurethanes and other advanced polymers. Society runs on plastics, and subtle tweaks from chemicals like TMP separate the stuff that cracks under pressure from the gear that lasts for years.
Another fascinating use shows up in the push for greener fuels and energy. TMP functions as a building block in the creation of stable organic radicals, notably TEMPO. This stuff lands in batteries and energy storage devices, where lifespan and reliability come before anything else. Researchers experiment with these systems, dreaming of powering entire neighborhoods off the sun and wind. TMP sits upstream, quietly forming part of those dreams.
Beyond batteries, certain air filtration and oxidation processes depend on TMP-derived catalysts. Think air purification for big cities or delicate electronics factories—places where precise chemistry can mean cleaner air, safer products, and fewer sick days for workers.
All these applications don’t come without challenges. TMP production generally involves sensitive steps and hazardous chemicals, not the kind you want spilled or mishandled. People care about how industry workers stay safe and how much waste ends up in the landfills. Some companies have started investing in greener synthesis routes, attempting to lower the environmental footprint from start to finish. This trend doesn’t stem from checklists; it grows from real-world problems—disposal costs rising, new regulations cropping up, reputations on the line.
If folks want to keep benefiting from advanced materials and cleaner energy, it’s worth keeping an eye on how compounds like TMP get made and handled. Every little improvement upstream often brings surprising rewards downstream, from safer jobs to cleaner air.
| Names | |
| Preferred IUPAC name | 2,2,6,6-Tetramethylpiperidine |
| Other names |
TMP 2,2,6,6-Tetramethylhexahydropyridine Hexamethylpiperidine |
| Pronunciation | /ˌtɛtrəˈmɛθəlˌpɪpəˈriːdiːn/ |
| Identifiers | |
| CAS Number | 768-66-1 |
| 3D model (JSmol) | `3Dmol.js:loadMolData("data:chemical/x-jmol;model=1;padding=2Jsmol2,2,6,6-Tetramethylpiperidine")` |
| Beilstein Reference | 1699790 |
| ChEBI | CHEBI:38471 |
| ChEMBL | CHEMBL1276 |
| ChemSpider | 10415 |
| DrugBank | DB04161 |
| ECHA InfoCard | 13c1c35e-8746-486a-a92c-b335e5e4e49e |
| EC Number | 206-134-9 |
| Gmelin Reference | 61720 |
| KEGG | C06344 |
| MeSH | D013706 |
| PubChem CID | 11839 |
| RTECS number | **EJ6300000** |
| UNII | 7J779O61G3 |
| UN number | UN1326 |
| CompTox Dashboard (EPA) | DTXSID7021564 |
| Properties | |
| Chemical formula | C9H19N |
| Molar mass | 143.28 g/mol |
| Appearance | White solid |
| Odor | amine-like |
| Density | 0.862 g/mL at 25 °C(lit.) |
| Solubility in water | slightly soluble |
| log P | 1.90 |
| Vapor pressure | 0.91 mmHg (25 °C) |
| Acidity (pKa) | 11.1 |
| Basicity (pKb) | 11.0 |
| Magnetic susceptibility (χ) | -9.59 × 10⁻⁷ cm³/mol |
| Refractive index (nD) | 1.453 |
| Viscosity | 19.81 cP (20°C) |
| Dipole moment | 1.18 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 234.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -107.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4097 kJ/mol |
| Pharmacology | |
| ATC code | D07AX |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H228, H302, H314 |
| Precautionary statements | P210, P261, P264, P280, P301+P312, P304+P340, P312, P330, P305+P351+P338, P405, P501 |
| NFPA 704 (fire diamond) | 1-3-0 |
| Flash point | 50 °C (122 °F; 323 K) |
| Autoignition temperature | 410 °C |
| Explosive limits | 3.6–10.9% |
| Lethal dose or concentration | LD50 (oral, rat): 1400 mg/kg |
| LD50 (median dose) | Oral rat LD50: 2000 mg/kg |
| NIOSH | SKC06550 |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 10 ppm |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
2,2,6,6-Tetramethylpiperidinyloxy (TEMPO) N,N-Dimethylpiperidine Piperidine 2,6-Dimethylpiperidine 2,2,6,6-Tetramethylpiperidone N-Methylpiperidine |