2,2,6,6-Tetramethylpiperidin-4-ol sprang from breakthroughs in amine synthesis during the 20th century, as researchers experimented with ways to lend thermal stability and radical resistance to organic molecules. Interest in hindered amine structures, both from academic labs and the chemical industry, grew quickly after the discovery that the bulky methyl groups at the 2 and 6 positions kept the molecule from oxidative breakdown. This property paved the way for the development of related compounds, including the well-known radical scavenger TEMPO, a close structural cousin. When chemical manufacturing scaled up after the 1960s, major suppliers of amines and specialty chemicals worked their way into the synthesis, tailoring the base piperidine framework into various functional forms for both research and commercial applications.
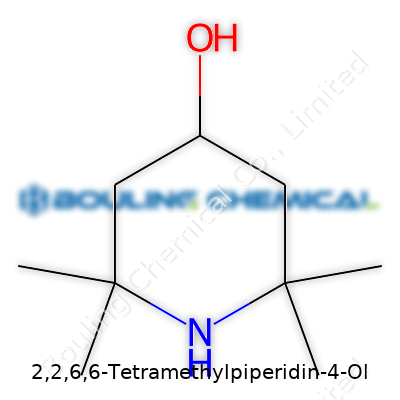
2,2,6,6-Tetramethylpiperidin-4-ol stands as a nitrogen-containing heterocycle, sporting four bulky methyl groups that cluster around the piperidine ring. Its structure lends the molecule both steric protection and high volatility resistance. This alcohol finds its way into labs and industrial facilities across the globe, sought after for its unique profile—serving as a building block, antioxidant, and an intermediate in a wide range of syntheses. Chemists often reach for this molecule when seeking stability under heat or oxidative conditions, and materials scientists value its role in creating advanced polymers and stabilizers.
A colorless, crystalline solid or oily liquid at room temperature, 2,2,6,6-Tetramethylpiperidin-4-ol holds up under moderate heat thanks to those methyl groups. It brings a sharp, amine odor typical of many smaller nitrogen heterocycles, dissolves well in organic solvents like toluene, ether, and chloroform, but stays stubbornly out of water. Melting point ranges can stretch, though many batches hover around 50-57°C, and the boiling point usually lands near 220°C under atmospheric pressure. A molecular formula of C9H19NO and a molar mass of about 157.25 g/mol round out the physical profile. Chemical stability stands out as one of its prime attractions, especially its resistance to autoxidation, which keeps it useful across many temperature and storage conditions.
Suppliers sell 2,2,6,6-Tetramethylpiperidin-4-ol in high purity grades, commonly at 98% or above, with clear documentation of impurity profiles—any deviations in water content or alternative piperidine derivatives show up in analysis certificates. Labels highlight storage advice—cool, dry, and well-ventilated spaces away from oxidizers or acidic compounds—reflecting both volatility concerns and the tendency for amines to react with strong electrophiles. Industrial packaging comes in tightly sealed drums or specialty bottles, often lined for long-term stability. Every shipment includes hazard statements for skin and eye irritation, flammability warnings, and a recommendation to use in well-ventilated lab spaces or under fume hoods. Date of manufacture and batch tracking give researchers confidence about shelf life and consistency.
Most synthesis routes for 2,2,6,6-Tetramethylpiperidin-4-ol start with 2,2,6,6-tetramethylpiperidine, itself made from acetone and ammonia derivatives in multi-step processes, then introducing a hydroxyl group at the 4-position by selective oxidation or nucleophilic functionalization. Some methods call for catalytic oxidation under carefully controlled air or peroxide conditions; others use Grignard reagents to install the alcohol group in a more targeted fashion. Yields improve when the process takes place at low temperatures and under anhydrous conditions, as excess water threatens both purity and overall conversion rates. Factories running these syntheses work in closed systems with nitrogen atmosphere to prevent side reactions, and scale efficiently by recycling solvents and minimizing byproducts.
2,2,6,6-Tetramethylpiperidin-4-ol can undergo further functionalization at the alcohol group, allowing esterification, etherification, or oxidation to form N-oxyl radicals, much like the transformation into TEMPO. The core piperidine structure holds up well under both acidic and basic conditions, and those methyl groups at the 2 and 6 positions stop reactions that would usually destabilize a plain piperidine ring. Chosen reaction partners range from acyl chlorides to isocyanates, and the resultant esters, amides, or carbamates often find value as advanced intermediates in the pharmaceutical, agrochemical, and polymer sectors. Chemists often modify the alcohol site to tune solubility or thermal properties, leading to new classes of performance additives for high-tech materials.
Some folks refer to this compound by the shorthand TMP-OH or provide trade names from major suppliers. Among other synonyms, “4-Hydroxy-2,2,6,6-tetramethylpiperidine,” “2,2,6,6-Tetramethyl-4-piperidinol,” or “Tetramethylpiperidinol” shows up on safety data sheets and in patent filings. These alternate names reflect differences in chemical indexing and supplier preferences, but the backbone structure remains consistent. Researchers swapping protocols need to watch for these synonyms to avoid costly purchasing mistakes.
Working with 2,2,6,6-Tetramethylpiperidin-4-ol means following safety standards much like other small amines—wearing gloves, eye protection, and working in a ventilated space. Direct contact causes skin and eye irritation, and inhalation of dust or fumes sends users running for fresh air. Facilities using large amounts maintain eyewash stations, keep spill control kits on hand, and keep detailed records of use and disposal. Local environmental rules require correct labeling and waste tracking, since amine-containing compounds sometimes draw attention from regulatory agencies. Training new staff on emergency procedures and correct use of PPE makes a difference, especially where inexperienced students or large-scale production lines meet.
Beyond its basic role as a synthetic intermediate, 2,2,6,6-Tetramethylpiperidin-4-ol strengthens the backbone of polymer stabilizers used in fiber optics, plastics, rubbers, and coatings; its resistance to oxidation and thermal breakdown makes it a mainstay for long-lasting outdoor materials. Researchers use it to build stable free radicals for organic synthesis, and in the lab, it sometimes acts as a blocking or scavenging agent for challenging reagents. Pharmaceuticals also draw on this molecule’s structure for biocompatible intermediates, with certain derivatives advancing to late-stage trials. Electronics and lithography processes benefit from its steric stability, often using it as a template or blocking group in microfabrication.
Scientists and product developers keep pushing into new territory with 2,2,6,6-Tetramethylpiperidin-4-ol, especially as the need grows for advanced stabilizers in tough applications like photovoltaic panels, automotive coatings, and life sciences. Recent research investigates improved catalytic processes for conversion to key derivatives; academic groups focus on mechanistic insights and radical chemistry unique to this structure. Industry-scale R&D aims for eco-friendlier processes, less waste, and efficient recycling of byproducts. Researchers at specialty chemical firms often develop new blends based on the tetramethylpiperidine core, seeking unique ways to impart longevity and resistance to harsh environments.
Toxicological studies place 2,2,6,6-Tetramethylpiperidin-4-ol at moderate risk levels for acute exposure, noting skin and eye irritation as primary concerns. Animal studies and cell cultures focus on effects from repeated exposure, with results guiding workplace safety standards and disposal practices. Breakdown products from incineration or long-term storage attract regulatory scrutiny, pushing manufacturers to publish new data and improve documentation. Ongoing independent research monitors for any bioaccumulation or long-term health effects, ensuring community safety around both manufacturing plants and research labs. As with most amine-based intermediates, awareness of cumulative exposure risk and safe storage makes a difference for staff health outcomes.
Looking ahead, demand for stable, high-performance chemical building blocks gives 2,2,6,6-Tetramethylpiperidin-4-ol a sturdy market foothold. Its bulk and steric protection suit it for research into new classes of antioxidants, long-lasting polymers, and next-generation pharmaceuticals. The trend toward sustainable and green synthesis also puts pressure on companies to refine manufacturing and purification methods, focusing on reduced solvent use, energy consumption, and hazardous byproducts. With consumer and regulatory environments tilting toward safer, longer-lived materials, investment keeps flowing into new derivatives and improved safety profiles. Lighter environmental impact and broader biocompatibility look set to push this compound into even wider fields, from medicine to electronics to materials science.
People often look at chemical names like 2,2,6,6-Tetramethylpiperidin-4-ol and think, “That sounds intimidating.” In reality, it’s a tool quietly playing a big role in materials science and manufacturing. For those who’ve spent any time in a research lab or involved with product formulation, the mention of such compounds brings back the sharp tang of solvent, glassware, and a sense that something’s getting done—usually something important for protecting modern materials from breaking down.
This compound acts as a stabilizer inside plastics and polymers, especially the kind of plastics that face a lot of sun or oxygen exposure. Most consumers never notice it, but anyone who has watched a cheap lawn chair crumble in the sun knows what goes wrong when you skip proper stabilizers. 2,2,6,6-Tetramethylpiperidin-4-ol helps plastics last longer by catching free radicals that start forming when ultraviolet light or oxygen starts attacking the material. This kind of chemistry keeps things like outdoor furniture, car parts, and packaging from breaking down too quickly.
In my own time in a lab, we handled plastics meant for car dashboards. Customers didn’t want faded or brittle interiors just a couple summers in. Including stabilizers like this one could mean the difference between a happy customer and a warranty headache.
Another use pops up in chemical synthesis. 2,2,6,6-Tetramethylpiperidin-4-ol plays a supporting role when chemists look to build more specialized molecules. As a precursor, it can be turned into other useful compounds or even serve as a base for further modification. Synthetic chemistry can sound abstract, but it’s how new drugs, better coatings, and more durable plastics start out. Every new ingredient needs to be reliable and predictable, especially in labs focused on pharmaceuticals or specialty materials.
Chemical additives always draw questions about safety. Researchers spend real time deciding how much to use, where it ends up, and whether there’s a risk to people or animals. 2,2,6,6-Tetramethylpiperidin-4-ol, like many stabilizers, needs careful handling. Factories and recyclers both look at how these additives break down in the environment or if they stick around. Balancing performance with safety and sustainability takes more than just following regulations; it’s about respecting the responsibility for public health and future generations.
A lot of the answers come from developing greener chemistry and being smart about product design. Sometimes that means finding similar additives with faster environmental breakdown, or working on robust recycling streams that keep additives from leaking out of old materials. Pushing ahead with research, funding safer options, and doubling down on recycling stands as the real way forward. If the industry and regulators both take lessons from past failures and stay proactive, the products touching everyday life can keep getting better without hidden costs down the road.
2,2,6,6-Tetramethylpiperidin-4-ol, known for its role in specialty chemistry, looks innocent compared to harsher industrial chemicals, but experience around labs quickly teaches respect for every bottle on the shelf. A clear label signals more than a name: it means rules apply, even if the hazards aren’t obvious at a glance. This compound can irritate skin, eyes, and even the lungs. Someone in a lab coat once wiped an unknown powder from a bench and spent the day dealing with rashes — that stuck with me throughout my research days.
Handling this compound without gloves, goggles, or a lab coat puts you one mistake away from trouble. Splashing is rare but not out of the question. Nitrile gloves protect skin. Well-fitted goggles keep accidental splatter away from your eyes, and a coat shields regular clothes from contamination. I’ve seen easygoing coworkers relax these habits and regret it — nothing slows down a research day like a chemical burn or a trip to health services.
Most labs rely on a good fume hood when pouring or mixing powders like this. Even if 2,2,6,6-Tetramethylpiperidin-4-ol doesn’t carry a strong smell, fine particulates can fly up during weighing, or solvents used with it turn into harmful vapors. Spending hours in tight spaces with poor airflow increases the risk of long-term exposure, a lesson that usually comes too late. I’ve worked in converted storage rooms with just a cracked window — never again.
Spilling chemicals happens to everyone, even after years of careful work. With this compound, a spill means cleaning right away, using tools meant for chemical cleanup and never scraps from the supply closet. Small containers help — fewer chances of a disaster from a dropped bottle. Storage in a dedicated dry cabinet with clear labeling keeps things straightforward for the entire team. Heavy lids, not flimsy stoppers, stop accidents in shared fridges.
Once, I saw a student pour leftover chemicals down a sink, ignoring guidance to use the waste bins. That shortcut never ends well. This compound joins organic waste containers, not mop buckets or public drains. Labs track disposal for a reason — not following it risks fines and health hazards far from the lab. Proper disposal keeps a clean record and a clear conscience, something every supervisor should want from their crew.
No piece of gear or protocol works alone. Regular training and an environment where even the new assistant can speak up make the big difference. Safety signs fade into the background without reminders and real support. I remember the stories and the mentors who did more than lecture; they asked questions and paid attention when someone faltered. That attitude builds habits that carry beyond any single bottle or procedure.
Modern labs benefit from digital inventory systems, recorded training sessions, and peer-to-peer oversight. Encouraging questions saves more trouble than any single piece of gear. If everyone expecting to handle 2,2,6,6-Tetramethylpiperidin-4-ol understands both the risks and the ways out of trouble, the job runs smoother and stays safer. In my time, nothing beat a culture where no one hesitated to double-check — trust and caution work together.
People don’t usually talk about 2,2,6,6-Tetramethylpiperidin-4-ol outside of a lab, but anyone who’s handled this compound learns a thing or two about how awkward it behaves in water. The molecule throws a jumble of methyl groups around a nitrogen ring. Just one glance at its structure and you spot trouble for mixing with water — those bulgy methyls resist getting cozy with polar solvents.
If you drop some of this chemical in water, you won’t see quick dissolving like with table salt or sugar. Instead, most stays stubbornly separate. An old-timer chemist might compare it to oil droplets bobbing up in a glass. The root: its nonpolar nature. Water likes sharing space only with others who bring along some charge, a polar face, or something that can hydrogen bond deeply. Sure, there's a hydroxyl group sticking out on that ring. In plain terms, a single -OH doesn’t outweigh four methyl groups hogging up the real estate.
Plenty of folks overlook why “will this dissolve?” pops up so often in research or production. At a pharmacy, major drug decisions rest on this fact. No scientist wants to work backwards after finding out a helpful compound won’t mix with water, blood, or cell media. Water is everywhere in living things. Labs always need to know if a compound can navigate these environments.
With 2,2,6,6-Tetramethylpiperidin-4-ol, the solubility problem pops up for every team trying to use it as an organic synthesis intermediate, a radical scavenger, or a stabilizer. Tossing the powder straight into water just wastes time and money. I’ve seen many projects stall for days until someone at the bench sniffs out a creative workaround.
Faced with low solubility, smart folks don’t just give up. Most switch to organic solvents like ethanol, acetone, or DMSO. These solvents feature in nearly every synthetic protocol involving touchy molecules. At small scale, dissolving 2,2,6,6-Tetramethylpiperidin-4-ol in DMSO solves plenty of headaches. This lets you add tiny amounts to biological or chemical mixtures — just enough to do its job, without turning your whole mixture into soup.
Some researchers build derivatives or tweak protocols to nudge that ring toward better water compatibility. Add an extra polar group, change the buffer, play with pH, or engineer carrier systems. Encapsulation into cyclodextrins or nanoparticles, for example, can sneak stubborn chemicals into aqueous environments. Over time, these tricks help open up new applications no one would dream about if stuck dealing only with insoluble powders.
It’s easy to shrug off solubility as just another line on a safety data sheet, but chemical handling sits right at the core of any bench work. Even a boring physical property like water solubility changes the pace of discovery and the cost of production. When it turns out that a molecule resists water, the best labs get stubborn and push through the challenge. It comes down to swagger, resourcefulness, and a deep bench of chemical know-how. That honesty about physical properties, shared from one scientist or engineer to the next, sets up the next breakthrough. If you’ve watched old chemistry hands navigate this one, you get why no one forgets their first lesson about what really happens at the bottom of a beaker when you combine oil-like molecules with clear water.
Ask anyone working in an organic lab about 2,2,6,6-Tetramethylpiperidin-4-ol, and chances are you’ll hear a story about stable nitroxides or protective chemistry. It stands out among piperidine derivatives, not just for its structure, but for how it pops up everywhere from antioxidant research to polymers. With so much attention, people naturally want to pin down its molecular formula. The answer, after counting the carbons, hydrogens, nitrogens, and oxygens, rings clear as C9H19NO.
Let’s picture what this formula means. The piperidine ring forms the backbone—a six-membered nitrogen-containing ring, which is a classic scaffold in synthetic chemistry. Four methyl groups cling to positions two and six. Chemistry students like me have spent hours drawing all those methyls around the ring, and it’s almost like decorating a holiday tree with ornaments—they go in predictable spots, but each placement matters. Then at the four position, a hydroxyl group sticks out. Adding it all up, you find yourself counting nine carbons, nineteen hydrogens, one nitrogen, and one oxygen.
The details might look dry, but knowing the formula lets chemists do more than just recite facts on an exam. It allows for proper calculation, whether you’re weighing out reagent or planning a synthesis. Mistakes in formulas don’t just waste chemicals—they often waste your afternoon, too. When I worked in a polymer lab, projects depended on exact molecular weights. Misplacing a methyl or ignoring an oxygen meant wrecked polymer chains or off-target products. The formula, C9H19NO, gives folks at the bench the confidence to proceed, knowing their reactions make chemical sense.
2,2,6,6-Tetramethylpiperidin-4-ol comes up in research around free radical chemistry and stabilization. Antioxidants built on its backbone resist breaking down under stress. Polymers gain extra durability when these stabilizers sit among their chains. I’ve sat in on meetings where researchers debate the best substitutions at stubborn positions, and everything comes back to how the core formula stays intact despite all the modifications. Journals cite this molecule in studies of oxygen reactivity, where its particular structure shields other molecules from unwanted oxidation. The benefit is both practical and scientific: longer shelf life for plastics and more predictable lab reactions.
Learning the molecular formula is the start, not the finish. Labs that depend on this compound benefit from clear protocols. Step one always asks for the formula, just as a carpenter checks the length of a board. Raw data in the form of C9H19NO then drives choices about solvents, reaction temperatures, and purification techniques. Spotting small errors early on comes from sharing knowledge and keeping up with published data. Educators and team leads share best practices—not just to teach, but to save time and money in the lab.
Improvement comes when teams talk openly about mistakes. If someone miscalculates the formula, retraining is more effective than blame. Open databases stocked with accurate molecular data can help everyone, from seasoned researchers to early undergrads. More robust labeling and reference materials make a difference. I've watched even senior chemists double-check their work thanks to a bolded formula on a reagent jar. Simple fixes help avoid repeat errors and keep research moving forward.
In my early days working in a university chemistry lab, storage never got much attention—until a chemical leaked through a loose cap and ruined some notes and a keyboard. Small moments like that taught me to never underestimate the impact of how a compound is stored. With 2,2,6,6-Tetramethylpiperidin-4-ol, the lessons stack up quickly. This compound, known for acting as an antioxidant and stabilizer in organic synthesis, wants a safe and stable home. Failing to provide that puts researchers at risk of inhaling harmful vapors, and it can even affect product quality in industrial settings.
This compound stands out thanks to those four methyl groups. They bulk up the molecule’s structure, making it relatively stable but not untouchable. Even stubborn chemicals react to sunlight and oxygen over time. The structure puts off a faint amine odor, and if it evaporates or spills, inhalation risks go up. Long exposure irritates the skin or eyes. Some of my peers dealt with minor rashes or headaches during hasty cleanups, which says enough about proper protocols.
Glass bottles with tight-sealing screw caps work best. Plastic containers may leach, swelling studies back that up. Even a clear bottle can be a mistake. Amber glass reduces light exposure, cutting the risk of degradation. Labels matter—no scribbles or chemist shorthand. Clear hazard symbols and the full name keep everybody in the loop, from new interns to visiting auditors.
Molecules like 2,2,6,6-Tetramethylpiperidin-4-ol want their temperature steady and cool. Stick them in a general chemical cabinet at room temperature (15-25°C), away from direct sunlight and any heat source. If storage sits near a radiator or under strong lamps, bring in thermometer stickers or digital loggers. Moisture brings headaches, too. Humidity will increase clumping or lead to slow hydrolysis. I’ve seen desiccants—like silica gel packs—used in cabinet drawers to keep things dry. If the lab sits in a humid city, that little step pays off, keeping the compound free-flowing and active for longer.
This compound won’t explode on contact with air, but trouble brews around oxidizers or acids. An old co-worker almost caused a hazmat call storing this near a ferric chloride bottle. Segregate from strong oxidizing chemicals, mineral acids, bases, and anything labeled peroxide-former. Shelves should follow this rule and not rely on a “grouped by function” approach—this means separate those stabilizers from pools of solvents, acids, and cumulatively reactive agents.
In every lab or facility I’ve worked, lockable cabinets for specialty chemicals keep out wandering hands. Regular inventory checks stop the buildup of forgotten, outdated materials. I once found a five-year-old bottle in a forgotten box, yellowed and thickened, lost to neglect—hazardous waste by that point. Keeping accurate logs prevents these scenarios, saves money, and limits unnecessary risks.
Sometimes storage rules sound like overkill, but they get written in response to real-world accidents and oversight. Giving this compound the right home ensures safer labs, less waste, and stronger results. Simple choices—dark bottles, separate shelves, trustworthy logs—make the task routine instead of risky. In bigger operations, a regular walk-through from safety officers keeps these habits alive, proving that daily caution shows its value over time.
| Names | |
| Preferred IUPAC name | 2,2,6,6-Tetramethylpiperidin-4-ol |
| Other names |
TMP-OH 4-Hydroxy-2,2,6,6-tetramethylpiperidine 4-Hydroxy-TMP 4-Piperidinol, 2,2,6,6-tetramethyl- |
| Pronunciation | /ˈtɛtrəˌmɛθəlˌpɪpəˌrɪdɪnˌfɔːr.ɒl/ |
| Identifiers | |
| CAS Number | 1065-48-3 |
| 3D model (JSmol) | `3DStructure:JSmol/organic/2,2,6,6-Tetramethylpiperidin-4-ol` |
| Beilstein Reference | 1209245 |
| ChEBI | CHEBI:92483 |
| ChEMBL | CHEMBL138012 |
| ChemSpider | 164014 |
| DrugBank | DB08230 |
| ECHA InfoCard | 01-2120766593-38-0000 |
| EC Number | 1.1.1.8 |
| Gmelin Reference | 113174 |
| KEGG | C03316 |
| MeSH | D04719 |
| PubChem CID | 10410 |
| RTECS number | YV0350000 |
| UNII | 1P2T54F180 |
| UN number | UN3272 |
| CompTox Dashboard (EPA) | DTXSID8084609 |
| Properties | |
| Chemical formula | C9H19NO |
| Molar mass | 157.27 g/mol |
| Appearance | White to pale yellow solid |
| Odor | Odorless |
| Density | 0.934 g/cm³ |
| Solubility in water | slightly soluble |
| log P | 1.18 |
| Vapor pressure | 0.04 mmHg (25 °C) |
| Acidity (pKa) | 20.1 |
| Basicity (pKb) | pKb = 4.47 |
| Magnetic susceptibility (χ) | -88.8e-6 cm³/mol |
| Refractive index (nD) | 1.463 |
| Viscosity | Viscosity: 14 mPa·s (at 20 °C) |
| Dipole moment | 2.53 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 296.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -327.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -6147.6 kJ/mol |
| Pharmacology | |
| ATC code | D02BX06 |
| Hazards | |
| Main hazards | Causes skin irritation. Causes serious eye irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302 + H312 + H332: Harmful if swallowed, in contact with skin or if inhaled. |
| Precautionary statements | Precautionary statements: P210-P280-P305+P351+P338-P337+P313 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | > 108 °C |
| Autoignition temperature | 270 °C |
| Lethal dose or concentration | LD50 Oral Rat 2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 2500 mg/kg |
| NIOSH | SR2945000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 2,2,6,6-Tetramethylpiperidin-4-Ol: Not established |
| REL (Recommended) | 24 months |
| Related compounds | |
| Related compounds |
2,2,6,6-Tetramethylpiperidine 2,2,6,6-Tetramethylpiperidone TEMPO (2,2,6,6-Tetramethylpiperidin-1-yl oxyl) 4-Hydroxy-TEMPO 2,6-Dimethylpiperidine |