Chemists first synthesized 2,2,6,6-Tetramethyl-4-Piperidylamine in the drive to enhance stability for industrial applications. Early on, it caught attention as a derivative within the piperidine family, recognized mostly for its robust resistance to oxidative breakdown. Over recent decades, the chemical has gone from a niche research molecule to a staple in laboratories and production lines, solidifying its place thanks to its unique steric hindrance and resulting reactivity profile. As technologies and synthetic methods advanced, better yields and purities became possible, propelling use forward in coatings, polymers, and electronics.
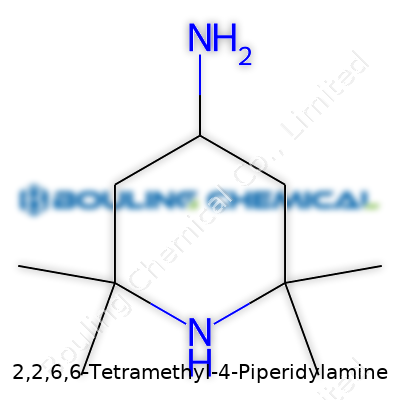
2,2,6,6-Tetramethyl-4-Piperidylamine carries the formula C9H20N2, displaying a compact six-membered ring structure with four methyl groups perched at the two and six positions, and an amine function standing out at the four position. This configuration lends bulkiness near the amine group, which greatly influences its chemical interactions and performance in stabilization tasks. You might find it on shelves labeled for laboratory synthesis, additives in advanced materials, or as a catalyst modifier.
This compound usually appears as a colorless to pale yellow liquid or crystalline solid, depending on the storage temperature and supplier. Its melting point ranges near 20°C, making it easy to handle at room temperature in liquid form. With a boiling point climbing past 200°C, it’s stable under most working setups. In terms of solubility, it mixes readily with organic solvents like ethanol, acetone, and ether but resists dissolving in water. Its basicity reflects the strong electron-donating nature of the four bulky methyl groups, which shield the nitrogen from unwanted side reactions, another reason for its frequent use where longevity under stress matters.
Manufacturers usually indicate purity levels above 98% for 2,2,6,6-Tetramethyl-4-Piperidylamine, and you’re likely to see warnings about moisture absorption since free amine groups can absorb atmospheric CO2 and water. Containers sport clear hazard and precautionary statements, often following guidance from OSHA and REACH. Storage information highlights the need for cool, dry spaces and sealed vessels, since the amine may react with acidic fumes. Measuring specifications, such as refractive index and density, appear on the typical material safety data sheets, matching results from most reputable chemical suppliers.
Synthesis often uses Robinson–Gabriel cyclization or similar cyclization of precursors like acetone and ammonia with methylation steps layered in. Practical laboratory setups convert starting amines into their tetramethylated forms over controlled heating, using well-established catalysts to direct the formation of the characteristic piperidine ring. Scale-up to industrial production demands careful control of reagent ratios and temperature to avoid by-products. In most factories, engineers take extra care around the methylation stages, since runaway methylation can introduce unwanted tars and alter product purity.
One of the chemical’s most intriguing behaviors comes from the way the crowded 2 and 6 methyl groups block unwanted attacks from electrophiles or nucleophiles, allowing selective transformations at the amine site. Functionalization usually targets the nitrogen atom, making coupling with acids, chlorides, or other reactive intermediates straightforward. More advanced chemistry leverages the molecule’s bulk in the creation of hindered amine light stabilizers (HALS), as the ring can anchor a wide variety of functional moieties, dramatically expanding the palette for new materials research.
Catalogs list this molecule under several names: 4-Amino-2,2,6,6-tetramethylpiperidine, TMPDA, and sometimes its systematic designation, 2,2,6,6-tetramethylpiperidin-4-amine. In polymer additive markets, suppliers may use shorthand or proprietary blends incorporating this amine for antioxidant or stabilizer formulations. Using its Chemical Abstracts Service (CAS) registry number has become common for paperwork, ensuring clarity across international markets.
Because it carries strong amine functionality, direct exposure leads to skin and eye irritation for some handlers, demanding routine use of gloves, goggles, and fume hoods during laboratory work. Inhalation of vapors triggers discomfort in the respiratory tract, and safety data sheets press for immediate washing and medical consultation after accidental contact. Occupational guidelines point out the compound’s reactivity with oxidizers and acids, resulting in regular training refreshers and incident drills in facilities that use it. Regulatory organizations insist on proper labeling, record-keeping, and disposal protocols, aiming to prevent mishaps and environmental releases.
2,2,6,6-Tetramethyl-4-Piperidylamine keeps popping up in places where stability against heat and light counts. Engineers design HALS molecules from this backbone to shield paints, plastics, and coatings from sun and weather. Polymer scientists favor it as an additive that slows down photo-oxidative chain reactions without staining or changing visual properties. In electronics, it can protect polymers from yellowing under circuit board temperatures. Some research even explores its use in battery electrolytes, though this application needs more refinement before commercial markets follow suit.
Recent studies explore not just the chemical’s traditional stabilization role, but also its function as a building block for custom catalyst supports and responsive materials. Academic journals highlight its selectivity in post-polymerization modifications and new, highly efficient synthetic approaches that cut down on waste and energy consumption. I have seen collaborative projects between universities and manufacturers build entirely new classes of high-durability materials by cleverly tweaking the amine’s functional groups, which offers another reminder that strong relationships between theoretical chemists and process engineers drive practical advances.
Laboratories keep close tabs on both acute and chronic effects. Animal studies at moderate doses show reversible irritation and mild sensitization. Extended exposure at higher concentrations produces kidney and liver changes, making routine health monitoring vital for industrial workers. Environmental labs screen wastewater and process emissions for amine residues since they can stress aquatic life in downstream settings. Regulators in Europe and North America rely on cumulative datasets, regularly re-evaluating occupational exposure limits to keep workers and the environment safe.
As the demand for tougher and longer-lasting plastics, paints, and electronics grows, 2,2,6,6-Tetramethyl-4-Piperidylamine will likely stay in heavy rotation. Chemists will continue to develop safer downstream derivatives and more sustainable manufacturing routes, such as biocatalytic methylation or solvent-free reaction streams. Some engineers hope to further shrink waste and energy input, catching the wave of green chemistry. Interest in recyclable polymers and electronics keeps pushing the search for stabilizers that not only last but also break down harmlessly at end-of-life. Companies that can hit this mark with new derivatives of the base molecule may find themselves with an edge as environmental standards tighten.
Anytime a plastic garden chair sits out in the sun for too long, it starts to look tired and brittle. That’s ultraviolet light breaking chemical bonds, making the material weak and cracked. Years ago, yards filled up with faded lawn furniture because compounds with muscle against UV rays didn’t exist. This is exactly where 2,2,6,6-Tetramethyl-4-piperidylamine, often called TMPDA in labs, shines. It’s one of the building blocks for light stabilizers—specifically, hindered amine light stabilizers or HALS. By adding TMPDA derivatives to plastics, the material holds up against the harshest sunlight, whether in outdoor toys, greenhouse films, or car parts. In my experience at a plastics plant, swapping in a HALS additive based on TMPDA dropped complaints about yellowing plastics by half.
A home painted last year can look faded and dull before the second summer unless paint manufacturers step up protection against UV degradation. TMPDA goes into the formulation of stabilizers that help paints, varnishes, and coatings keep colors true and surfaces smooth. Paints with HALS additives hold onto pigments longer, especially on outdoor structures and cars. Paint engineers regularly include these stabilizers in their best outdoor formulas. Real-world testing on warehouses and outdoor decks showed less peeling and less discoloration after just one season when TMPDA-derived HALS joined the recipe.
Polyurethane foam makes cushions comfy and car seats safe, but UV can turn it into a crumbly mess. TMPDA-based products help toughen up polyurethanes for automotive interiors, insulation panels, and even shoe soles. The same chemistry pops up in adhesives and polyurethane-based coverings that have to handle sunlight, temperature swings, and rain. One furniture maker I spoke with said that after using a HALS system built around TMPDA, warranty calls about seat breakdown dropped, making both the business owner and customers happier.
Outdoor signs face relentless sun, wind, and rain. In practice, inks and elastomers start fading, crazing, or losing flexibility without stabilizers. Additives crafted with TMPDA keep billboards and labels popping with color. Rubber seals and gaskets rely on similar protective magic, holding their stretch and bounce, not just on factory shelves but after months exposed to weather. In production meetings, manufacturers focus as much on how their product stands up to the environment as any other feature, underscoring the value TMPDA brings to the table.
The world talks about recycling plastics, but no one wants recycled materials that crumble or discolor in sunlight. TMPDA boosts stability in recycled materials too. It holds up in mechanical recycling, where stabilizers face the challenge of old, sun-exposed feedstock. By improving recycled polymer longevity, more products can use recycled content without sacrificing performance, which cuts both landfill use and raw material demand.
Manufacturers look for safer, more sustainable additives every year. TMPDA-based HALS have a track record supported by regulatory reviews—as of now, they don’t tip over into any of the major health concern lists when used as directed. Companies keep running safety assessments, especially for food-contact items, but the track record so far gives confidence both inside industry and out.
Protecting products from sunlight and weather doesn’t sound glamorous, but practical chemistry like TMPDA saves billions of dollars in property and waste. Better plastics, coatings, foams, and inks mean longer life, less waste, and less frustration. That’s why I keep an eye on advances in this field—each improvement means a little less plastic in a landfill and a few more garden chairs that make it through another summer.
If you've worked in a lab, factory, or any kind of workshop, you know a chemical name like 2,2,6,6-Tetramethyl-4-Piperidylamine tends to turn heads and spark questions about what it actually does to people or the environment. I've had my share of moments handling unknown liquids and powders, double-checking my gloves, and hoping my safety goggles did their part. The truth is, chemical exposure rarely announces itself with a big warning sign. Sometimes the problems creep in quietly.
Plenty of industrial chemicals pack a punch, and this one lands firmly on the list of compounds that deserve a good deal of respect. It sits under the family of piperidylamines, known for strong basic properties. Human data is limited, and you won't find thousands of people lining up for voluntary exposure tests. What we do know comes from toxicity studies in animals, chemical structure clues, and how similar compounds behave.
According to safety data gathered from chemical suppliers and the European Chemicals Agency, 2,2,6,6-Tetramethyl-4-Piperidylamine can cause skin and eye irritation. If splashed directly onto skin or eyes, burning, redness, or more serious damage can follow. Breathing in its vapor or dust irritates the nose and throat. Accidental swallowing brings symptoms like stomach pain, nausea, or worse if a significant amount gets involved. It’s not listed as acutely lethal in small doses to humans, but just because it doesn’t lead the charts doesn’t mean it’s safe to take it lightly.
By comparison, other tetraalkylpiperidines have shown organ toxicity after repeated exposure in animal studies, especially affecting the liver and kidneys. Chronic effects aren’t well-documented, but the lack of evidence doesn't guarantee no risk—it sometimes means the substance hasn't gotten enough scrutiny. From my own experience, complacency with unfamiliar reagents usually leads to close calls or regrettable incidents.
In fields like plastics and coatings, this chemical shows up as an intermediate or stabilizing agent. If released into water, soil, or air during handling, concerns turn to fish, plants, or local groundwater. Persistence and breakdown rates aren’t thoroughly mapped, so it’s reasonable to err on the side of caution and keep it contained.
Wearing gloves, goggles, and a lab coat seem basic, but it’s surprising how often folks skip steps or cut corners. Proper ventilation and working near a fume hood don’t just protect individuals, they keep odors and vapors from surprising people down the hall. Disposal brings another key issue: dumping it into drains or regular trash can create downstream problems nobody wants to inherit.
We can't avoid using chemicals that carry hazards, but we can reduce the odds of something going sideways. Safety training that covers not just the “how” but the “why” makes a difference—most accidents I’ve seen happen where people treat a reagent with less respect than it deserves. Spills, allergic reactions, and long-term exposure often catch people off guard because they assume mild means harmless. Regular review of safety sheets and a culture that encourages asking questions goes further than posters and labels.
In the end, every compound with a tricky name hides a story of risk and responsibility. Respect in handling, care in disposal, and staying curious about long-term effects help protect both health and the planet from problems that linger long after a shift ends.
People who spend time in a chemistry lab can recognize the punchy name “2,2,6,6-Tetramethyl-4-Piperidylamine” as a piperidine derivative. Here’s what this mouthful boils down to. Picture a six-member nitrogen-containing ring. That’s the backbone. Four methyl groups crowd onto the ring—two at carbon 2 and two at carbon 6. You also find an amine group sticking out at the fourth carbon. Not a simple ring, but nothing outlandish. The result looks like a piperidine chair, decorated with balloons at four spots and a flag at the fourth. This configuration keeps the molecule rigid and evenly shaped, which can matter a great deal in both chemistry and industry.
The molecular formula of 2,2,6,6-Tetramethyl-4-Piperidylamine is C9H20N2. You get nine carbons, twenty hydrogens, and two nitrogens. Two nitrogens ring in as notable, one part of the backbone, and the other as that extra amine function. Chemists pay close attention here because two nitrogens in a compact scaffold give the compound potential for reactivity and versatility. Makers of light stabilizers prize this core for exactly that. In practice, these methyl “shields” can protect the ring’s properties, blocking oxidation and sunlight from breaking the molecule down too quickly. This keeps plastics and coatings from yellowing or weakening when exposed to the elements.
Years spent following chemical research make it clear why structure stories aren’t just for textbooks. Small changes, like the extra methyl groups stacked onto that piperidine ring, can change a molecule’s behavior by a mile. Bulky substituents like those set up a protective armor around the backbone. The result: more stability for polymer additives. I’ve seen these tweaks help extend the shelf life of construction materials, even sunscreen. Nitrogens in the structure often mean builders can link the molecule with other chains, or tweak its reactivity for new uses in UV stabilizers and antioxidants.
With any specialty molecule, the route to production, environmental impact, and downstream effects come up. Safety data for amines highlights careful handling—these aren’t benign substances in raw form. Industrial settings require good controls against leaks or spills, proper storage, and disposal routines. I’ve worked with teams handling similar amines, and strict adherence to materials safety data sheets isn’t optional if we want to avoid accidents or long-term health problems.
Making these piperidine derivatives often uses processes that generate waste, so attention to green synthesis methods picks up steam. Newer routes sometimes swap out harsh solvents for cleaner alternatives or use catalysts that trim down byproducts. Some companies also look into recycling used stabilizers or switching to more benign nitrogen sources. It's a work in progress—no magic bullet yet, but the drive to balance utility with responsibility continues to shape how chemists and manufacturers approach molecules like this.
In the search for safer, longer-lasting materials, leaning on structure–function relationships pays off. Research circles push for improved derivatives that combine the benefits of 2,2,6,6-Tetramethyl-4-Piperidylamine with better eco-profiles and less hazardous processing. Anyone working with these molecules eventually faces the same questions: Can we tweak the structure further, reduce system costs, and trim environmental impact without giving up the stabilizing effects? With transparent data, real-world testing, and broad collaboration, the answers will keep unfolding.
2,2,6,6-Tetramethyl-4-Piperidylamine doesn’t sound like something you’d find under the kitchen sink. It’s not. This amine shows up in labs, coatings, and polymer work. It packs strong reactivity and can create real trouble if people aren’t careful. I’ve watched people get too comfortable around these sorts of compounds. That’s when preventable accidents happen. Safety starts with understanding—this isn’t sugar or flour. Over time, you pick up a certain respect for volatile or potentially hazardous reagents, and this certainly counts as one of them.
Some folks want to shove every bottle into a cabinet and call it a day. Experience tells a different story. 2,2,6,6-Tetramethyl-4-Piperidylamine gives off corrosive fumes and can mess up plastics and metals if left exposed. Metal containers can rust or corrode, so use glass or HDPE instead. I always want a solid screw cap and a backup containment dish to catch any spills or leaks. Temperature matters too. Heat can speed up decomposition and bump up the pressure—store it cool, under 25°C, and way out of the sunlight.
If moisture finds its way in, the compound can react. Keep the bottle tightly closed and throw in a fresh desiccant pack. The best spot is a locked chemical cabinet, clearly labeled and away from acids or oxidizers. I’ve seen mixes go wrong because someone ignored the storage chart—you don’t want to clean up a chemical soup.
PPE can feel annoying, but I’d rather look awkward in goggles and thick gloves than deal with chemical burns. This amine burns skin and eyes and hits the lungs hard. Go heavy on the protection: splash goggles with solid side shields, a fitted lab coat, and gloves made for organics—nitrile usually works better here than latex. Stay away from open flames or heat sources; the fumes can drift fast and catch fire.
Work in a fume hood with steady airflow, not on an open bench. The stuff isn’t shy about releasing vapors. A seasoned lab tech once showed me how to keep all tools and wipes ready before even opening the bottle. That way you spend less time exposed, and spills get dealt with right away. No eating, drinking, or phone use around the material. Phone screens get greasy and are hard to clean, and no one wants to track residues out of the lab.
Even a small puddle needs quick action. Drenching with water isn’t the solution—this compound can react and make things worse. Use absorbent pads with the right chemical rating. Waste can’t go down the drain. Dedicated containers with the proper labeling keep the disposal folks safe—it helps everyone if the waste stream is clear and separated. Regular training in spill drills keeps reactions calm and responses quick. Don’t trust old habits; fresh eyes and reviewed protocols save headaches.
People forget that most incidents aren’t about chemistry—they’re about attention. Store chemicals where they can’t surprise anyone. Use protective barriers even when no one’s looking. Keep emergency washing stations within arm’s reach. In my experience, creating a habit of asking, “What could go wrong?” keeps teams safer. Share stories about close calls, and encourage reporting every minor spill or glove tear. Good habits and clear rules keep dangerous reactions inside the test tubes where they belong.
Every time the need for a specialty chemical like 2,2,6,6-Tetramethyl-4-piperidylamine comes up, the process involves more than just a quick internet search. Researchers, manufacturers, and labs looking to purchase in bulk know the difference between a quick retail solution and a dependable long-term supplier. Tracking the chemical’s availability means considering who’s stocking it, the purity level, price, delivery times, and—most importantly—safety and compliance. In the last decade, regulations around buying amines and other specialty chemicals have become more strict, which always weighs on procurement decisions.
Stores like Sigma-Aldrich, Alfa Aesar, and TCI America pop up on top of search results. These companies have built their reputations on supplying academic labs and industry, including for obscure amines and specialty reagents. For bulk purchases, direct negotiation with dedicated chemical distributors becomes essential. I've contacted companies like these over the years when sourcing acids, amines, or polymer additives, and the process always involves quote requests, material data sheets, and compliance paperwork—no quick add-to-cart.
Some distributors, especially those with a global reach like Merck and Thermo Fisher Scientific, can provide bulk volumes, sometimes stretching to drum quantities. High purity always comes with documentation, because auditing and safety checks are common at this scale. In my own experience working with mid-sized R&D labs, purchasing anything above a kilogram triggers a different workflow—one that brings compliance staff and environmental health into the loop. Security regulations on nitrogenous compounds and controlled chemicals enter the conversation here, slowing down procurement.
Taking shortcuts on sourcing leaves room for counterfeit products or misrepresented purity. Known distributors submit their products to verification programs like the American Chemical Society guidelines, which helps cut down on fraudulent listings. Dangerous precursors require proper tracking and safe transit. The cost per kilogram can seem high compared to bulk commodity chemicals, but the extra layers of safety, traceability, and service count for something in process-oriented environments.
On some occasions, local specialty chemical suppliers or custom synthesis labs can source or produce the compound in specific volumes. Finding a supplier with the technical ability to scale production safely makes a difference for both researchers and commercial ventures. Checking past client reviews or looking up regulatory registrations reduces risk. I learned early on that vetting a vendor via customer service interactions often gives away more about their professionalism than what a shiny website suggests.
Shipping rules change from country to country. Bulk chemical buyers in the United States, Europe, India, and China each face their own specific compliance frameworks—REACH, TSCA, or other local chemical control systems. I’ve seen projects delayed for months by customs checks and lack of accurate paperwork. Working only with suppliers who provide batch certificates and understand transit regulations prevents unpleasant surprises, and protects workers and the environment.
Procuring 2,2,6,6-Tetramethyl-4-piperidylamine in high volumes draws attention to quality, regulation, and reliability. Working with established distributors, asking for transparent paperwork, and following safety procedures ensures that chemical buyers end up getting the compound they ordered—safe, legal, and ready to use for real projects.
| Names | |
| Preferred IUPAC name | 4-Amino-2,2,6,6-tetramethylpiperidine |
| Other names |
TMPDA 2,2,6,6-Tetramethylpiperidin-4-amine 4-Amino-2,2,6,6-tetramethylpiperidine |
| Pronunciation | /ˈtuː,tuː,sɪks,sɪks-tɛtrəˈmɛθɪl-fɔːɹ-pɪˈpɛrɪdɪl.əˈmiːn/ |
| Identifiers | |
| CAS Number | 13635-74-8 |
| Beilstein Reference | 1818733 |
| ChEBI | CHEBI:84677 |
| ChEMBL | CHEMBL16460 |
| ChemSpider | 223682 |
| DrugBank | DB08357 |
| ECHA InfoCard | 03bfa7cb-9da5-4a5a-95e8-8bfb5bd7c3c2 |
| EC Number | 208-761-3 |
| Gmelin Reference | 136080 |
| KEGG | C06347 |
| MeSH | D04729 |
| PubChem CID | 10210 |
| RTECS number | YD0875000 |
| UNII | 6DH1W9VH8Q |
| UN number | UN3276 |
| CompTox Dashboard (EPA) | DTXSID2048344 |
| Properties | |
| Chemical formula | C9H20N2 |
| Molar mass | 170.29 g/mol |
| Appearance | White solid |
| Odor | Amine-like |
| Density | 0.896 g/mL |
| Solubility in water | Insoluble |
| log P | 1.38 |
| Vapor pressure | 0.04 mmHg (25°C) |
| Acidity (pKa) | 11.1 |
| Basicity (pKb) | pKb = 3.3 |
| Magnetic susceptibility (χ) | -8.55 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.483 |
| Viscosity | 15 mPa·s (20 °C) |
| Dipole moment | 2.27 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 344.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -39.3 kJ/mol |
| Pharmacology | |
| ATC code | D07AX |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin irritation, causes serious eye irritation, may cause respiratory irritation |
| GHS labelling | GHS02, GHS05, GHS07, GHS08 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H314, H317 |
| Precautionary statements | P261, P264, P271, P272, P280, P302+P352, P321, P362+P364, P304+P340, P312, P305+P351+P338, P337+P313, P332+P313, P333+P313, P363, P501 |
| NFPA 704 (fire diamond) | **NFPA 704: "2,2,6,6-Tetramethyl-4-Piperidylamine"** "2,2,0 |
| Flash point | 81 °C (closed cup) |
| Autoignition temperature | 252 °C |
| Lethal dose or concentration | LD50 Oral Rat 906 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral (rat) 906 mg/kg |
| NIOSH | RN3546 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 5 mg/m³ |
| Related compounds | |
| Related compounds |
2,2,6,6-Tetramethylpiperidine 2,2,6,6-Tetramethylpiperidin-4-ol 4-Amino-2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPAMINE) 2,2,6,6-Tetramethylpiperidine 1-oxyl (TEMPO) N-Methyl-2,2,6,6-tetramethyl-4-piperidylamine |