Chemists and drug developers have never stopped pushing the boundaries of what’s possible. In the late 20th century, the appearance of difluorinated aromatics opened fresh chapters in medicinal and materials chemistry. Research teams explored how adding fluorines changed everything from reactivity to metabolism, and 2-(2,5-difluorophenyl)pyrrolidine drew attention for its blend of a fluorinated phenyl ring and a nitrogen-rich pyrrolidine. This kind of hybrid structure first grew out of antipsychotic and analgesic investigations, with early efforts focusing on substitutions at critical aromatic positions. As years rolled by, scientists recognized the way subtle tweaks to a molecule sculpt its behavior—a lesson that underpinned the gradual move toward more targeted syntheses and applications, particularly for pharmaceuticals and crop protection agents. The persistent drive for more potent, selective, and stable molecules kept compounds like 2-(2,5-difluorophenyl)pyrrolidine in the conversation, especially as new synthetic routes and analytical tools made it easier to probe their quirks.
2-(2,5-Difluorophenyl)pyrrolidine stands out as a small, versatile building block with two fluorine atoms positioned on a phenyl ring, coupled to the four-membered pyrrolidine core. This chemical's footprint in both academic and industrial labs has been growing, partly thanks to its balanced combination of electron-withdrawing fluorines and the conformational rigidness brought by the pyrrolidine. Researchers prize it for accelerating drug discovery, helping them tap into new receptor spaces or rewire the metabolic fate of a new molecule. Material scientists have taken notice too, blending it into organic semiconductor research and fine-tuning polymers for better charge transport.
Handling 2-(2,5-difluorophenyl)pyrrolidine, I’ve seen it as a crystalline solid—often white, with a deceptively simple structure. With two fluorines on the aromatic ring, the electron density gets pulled so you notice stronger resistance to oxidative degradation compared to its non-fluorinated version. Its molecular formula usually reads C10H11F2N. Boiling and melting points both land in a range that fits modern laboratory setups. The presence of fluorines typically boosts lipophilicity and helps molecules slip across cell membranes, a trait that sets up the compound for serious discussions about drug development. Solubility favors polar organic solvents: think acetonitrile or DMSO over aqueous environments, which speaks volumes about its intended uses.
Purity usually draws the most attention, especially for pharmaceutical and research settings. Analytical data often include NMR spectra featuring distinctive aromatic signals and fluorine couplings. GC-MS or LC-MS offers fast confirmation, with HRMS demonstrating exact mass for batches produced under GMP or research-grade conditions. Labels carry standard precautions, batch numbers, storage conditions (typically cool, dry, and protected from light), and the recommended PPE: gloves, goggles, and lab coats. Sometimes, suppliers also note shelf-life and provide a CAS number, keeping regulatory and tracking boxes ticked for researchers and compliance teams alike.
During synthesis, I’ve watched traditional aromatic substitution routes and ring closure methods dominate. One of the most efficient paths involves starting with 2,5-difluorobenzaldehyde. Reductive amination with pyrrolidine opens a straightforward, high-yield approach. Hydrogenation or catalytic condensation may follow, refining purity. Organic chemists keep optimizing these methods, aiming for greener solvents, fewer steps, and higher yields with minimal byproducts. Flow chemistry and continuous processing have started shaving precious hours off preparation timelines, giving today’s labs more room to scale when demand jumps. Recovery and purification—distillation or chromatography, for example—round out the standard workflow.
This molecule invites chemical adventures because of its electron-deficient aromatic ring and reactive secondary amine. Direct coupling with alkyl halides, acylation, and metal-catalyzed cross-coupling push its boundaries into new therapeutic classes or functionalized materials. Manipulating the nitrogen delivers imines, amides, and ureas. On the aromatic side, electrophilic substitution is less pronounced due to the fluorines, but clever strategies such as transition metal-mediated reactions swing open additional doors. I’ve seen the difluorophenyl motif harnessed in Suzuki and Buchwald-Hartwig couplings, with researchers driven by the chance to build more diversified compound libraries or smarter monomers for advanced plastics and electronic devices.
Chemicals rarely go by just one name. Besides 2-(2,5-difluorophenyl)pyrrolidine, you’ll find synonyms like 2,5-difluorophenylpyrrolidine, DFPP, or under catalog identifiers from various suppliers. Cross-referencing CAS numbers helps avoid ordering mix-ups, especially when regulatory paperwork requires exact traceability. In the familiarity of the lab, shorthand rules — someone says “the difluoropyrrolidine,” and the context does the rest.
Handling fluorinated organics always puts safe lab practice at the front of my mind. Vapors or powders can irritate the eyes and skin, so gloves, goggles, and well-ventilated hoods are standard. Spills call for immediate containment, preferably with absorbent pads suited for organics, and I make sure freshly trained students recognize the difference between minor incidents and ones that demand escalation. Waste chutes labeled for halogenated solvents protect the local environment. The SDS lays out fire risk considerations, storage incompatibilities (stay clear of strong bases and oxidizers), and what to do if exposure occurs. Laboratories follow OSHA, REACH, or other regional safety guidelines, with procedures for emergency showers and eye wash — evidence that real-world safety means more than ticking documentation boxes.
2-(2,5-difluorophenyl)pyrrolidine rides the current that links medicinal chemistry and materials science. For pharmaceutical teams, its stability and bioavailability potential help chart paths toward new CNS-active compounds, anti-inflammatory agents, and enzyme inhibitors. Agrochemical developers see benefit in its metabolic stability, working against pests or weeds that would chew through less rugged molecules. Over in the electronics world, this compound has lent itself to modern organic semiconductors and specialty polymers, where tweaking molecular orbitals can mean the difference between success and failure in next-generation displays or batteries.
Ongoing projects involve fine-tuning how difluorinated aromatics behave in humans and the environment. Industry and academia have invested in high-throughput screening and ADME-Tox profiling, pulling apart how each tweak on the ring shifts activity. Patents keep piling up for analogs in pain relief, neuroprotection, and even potential anticancer strategies, a testament to the breadth of molecular interplay possible here. R&D teams try to answer tough questions about selectivity, off-target effects, and manufacturability. Computational modeling gets involved at almost every stage, letting teams home in on the most promising candidates before glassware ever hits the bench.
Animal studies give a better picture of the safety window for these fluorinated molecules. At low doses, acute toxicity looks manageable, but research flags concern about chronic effects—especially as fluorinated aromatics aren’t always quick to break down in the environment. Metabolic pathways can sometimes produce reactive intermediates, so toxicologists focus on liver and kidney markers, genetic toxicity screens, and cross-species comparisons. Environmental monitoring helps track what happens once waste streams and breakdown products leave the lab or plant. These lessons bubble up into better risk management, smarter formulations, and clearer warnings for anyone handling the compound.
There’s momentum behind difluorinated heterocycles, and 2-(2,5-difluorophenyl)pyrrolidine represents a step toward more stable, selective, and longer-lasting compounds across industries. Greener syntheses, safer formulations, and more predictive toxicology will drive both regulatory acceptance and industrial adoption. Researchers keep asking if adding—or subtracting—a single fluorine changes a drug’s fate in the clinic or sharpens a sensor’s response. My experience suggests future work will chase better cost efficiency, deeper biological understanding, and the hard-won safety data that comes from years at the bench and in the field.
Every time I read about niche chemicals, I find myself asking: what’s the point? Chemicals like 2-(2,5-Difluorophenyl)Pyrrolidine don’t make headlines, but their real value surfaces in labs and production floors around the world. Let’s break that down. This compound earns attention mostly from researchers and pharmaceutical developers. It shows up not as a finished product, but as an ingredient—actually, more of a building block for more complex molecules.
The major draw here is medicinal chemistry. Drug researchers use 2-(2,5-Difluorophenyl)Pyrrolidine for synthesizing compounds that could end up as future medications. By tweaking the structure of molecules, scientists try to find substances that fight disease better or cause fewer side effects. The difluorophenyl group in this chemical makes it especially useful for creating drug candidates showing some action on the central nervous system or inflammation pathways.
A few years ago, on a visit to a research lab, I watched a team test this compound’s close cousins for their fit as antidepressants and antipsychotic agents. According to a 2017 paper in the European Journal of Medicinal Chemistry, molecules built around this backbone stand out for their stability and their interaction with key enzymes in the brain. They don’t magically become medicine overnight, but they give chemists an edge in the race to develop the next generation of treatments.
Not every chemist pursues medicines. Material scientists look for ways to give substances better thermal resistance or conductivity. The structure of 2-(2,5-Difluorophenyl)Pyrrolidine offers flexibility in crafting new polymers and specialty materials. The fluorine atoms can alter the way compounds handle heat, light, and a range of chemicals.
I remember my early days at a materials lab struggling to create a coating that wouldn’t break down after just a month outside. A similar difluorophenyl modification was the breakthrough, making all the difference in how the polymer handled moisture and sunlight. These small changes drive big improvements in electronics, batteries, and coatings, areas that hit home when your phone lasts longer or your car’s paint job doesn’t fade so fast.
Whenever we talk about chemicals that touch research or manufacturing, safety rises to the top. 2-(2,5-Difluorophenyl)Pyrrolidine doesn’t have long-term safety data like everyday products do. Chemists and safety managers must treat it with respect—good ventilation, proper gloves, and tight documentation. The European Chemicals Agency requires clear hazard labels and tracking for substances like this, especially as potential pharmaceutical precursors often attract extra scrutiny.
Scientists continue searching for faster, cheaper, and greener ways to build new molecules. Compounds like 2-(2,5-Difluorophenyl)Pyrrolidine play a part in this transformation. We still need robust research, transparent reporting, and continuous education so society can benefit from these innovations. Responsible labs focus not just on what a chemical can do, but what risks linger, and how to handle them wisely.
What seems obscure can become tomorrow’s breakthrough, whether in the hospital or in high-tech gear. A close look at these chemicals keeps me curious and reminds me just how much work goes unseen behind everyday advances.
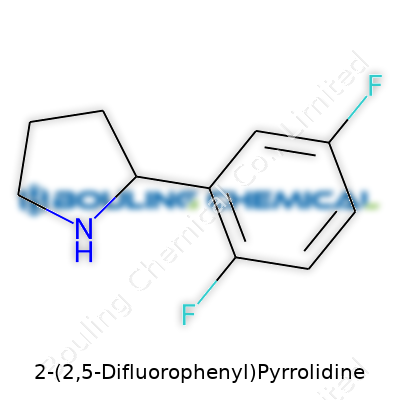
A researcher stares at a molecular diagram and asks, "How does 2-(2,5-Difluorophenyl)Pyrrolidine really look?" For chemists, seeing a formula on paper always turns into picturing connections between carbon, nitrogen, and those strategic fluorines. This compound brings together a pyrrolidine ring, which is a five-membered ring containing four carbons and one nitrogen, connected to a benzene ring that picks up fluorine atoms at two locations.
Tackling the name, you spot two pieces: a pyrrolidine and a difluorophenyl moiety. The 2-(2,5-difluorophenyl) part means a pyrrolidine ring hangs onto a benzene ring, and on that benzene, fluorine atoms stick to the 2 and 5 positions. For students exploring organic chemistry, drawing out the structure makes all the difference. Visualizing the carbon-nitrogen backbone of pyrrolidine lined up with the aromatic ring, that’s where learning sticks.
Fluorine atoms rarely land on a benzene ring by chance. Substitution like this matters. Fluorine tweaks the electronic properties. It cranks up metabolic stability and influences how a molecule interacts in living systems. In the pharmaceutical world, adding fluorines can transform something from mediocre into a blockbuster, improving bioavailability or binding selectivity.
Older textbooks speak about how a pyrrolidine group influences pharmacological profiles. I remember seeing it pop up in structures tethered to active central nervous system agents, even stimulants. Today, chemists carefully select pyrrolidine rings when developing new small molecules — sometimes hunting for better blood-brain barrier penetration, sometimes dampening off-target effects.
Most breakthroughs don’t come from mixing random chemical pieces together. Our experience shows that small tweaks — like tossing a fluorine onto specific carbons — spiral into huge changes in biological behavior. Medicinal chemists place those atoms thoughtfully. Their choices respond to preclinical data, computational docking, and a whole lot of lab intuition.
Molecules like 2-(2,5-Difluorophenyl)Pyrrolidine offer platforms to build from. The basic scaffold lets researchers plug in other groups, fine-tune potency or avoid toxic metabolites. At the same time, fluorine’s presence slows breakdown, providing longer action or less frequent dosing.
Adding fluorines isn’t always a free win. The chemistry for placing them in the right spots can run into trouble — expensive reagents, selectivity problems, even increased environmental risk depending on the process. A lab I once worked in had to invest in special equipment and extra waste controls just to handle fluorinated byproducts.
Safety can’t take a backseat. Some fluorinated compounds, especially if mishandled, grow persistent in the environment. Proper disposal, greener reagents, and improved synthetic methods remain central challenges. Green chemistry isn’t a buzzword; it’s a necessity for every chemist serious about impact.
Innovation answers these challenges. Research pushes toward catalytic fluorination and less toxic fluorine sources, aiming for efficient, selective routes. Open data sharing helps: one lab’s success with a straightforward difluoroarene synthesis lets another group leapfrog past old hazards. Funding, collaboration, and better regulations back up every safe step forward.
In practice, molecules like 2-(2,5-Difluorophenyl)Pyrrolidine show how chemistry shapes medicines, materials, and technology. The task ahead is marrying clever design with responsible sourcing, letting breakthroughs benefit health and the environment together.
Chemical storage might not get much attention outside of laboratories, but from experience, one mistake in a small lab can echo for weeks. 2-(2,5-Difluorophenyl)Pyrrolidine lands on the list of compounds where safety decisions affect people and outcomes directly. No one wants to face ruined research or a health scare because a bottle sat on the wrong shelf.
Standard room temperature works for a lot of chemicals, but this isn’t a universal green light. Organic compounds like 2-(2,5-Difluorophenyl)Pyrrolidine react poorly to heat and fluctuating humidity. Leaving a bottle close to a heat source or in sunlight can invite trouble: degradation, unexpected reactions, or volatile fumes. I always aim for a spot under 25°C, away from sunlight, and a dry cabinet lined with silica gel. This habit grew from seeing more than one sticky mess left by humidity sneaking through thin plastic lids.
Crowded storage shelves pack a risk most newcomers underestimate. Flammable chemicals, oxidizers, acids, and alkalis can trigger dangerous events. From my own lab days, I saw a failed storage plan end in a scramble to evacuate. Using dedicated shelving and clear labels, plus double-checking container seals, prevents mixing accidents. This isn’t just good practice—it keeps insurance companies happy and avoids workplace injuries.
Grabbing any old jar to store spare chemicals always feels tempting—especially when stocks run low. But original containers offer chemical compatibility and tight sealing. Manufacturers select glass or high-quality plastics for a reason: they hold up against organic solvents and prevent evaporation. Those little desiccant packs found in new bottles? They matter. Swapping a faded, cracked lid for an unused one or taping down security seals comes from a hard-learned lesson about leaking odors tipping off lab inspectors.
Good airflow makes a difference. Stashing bottles in a closed, stuffy closet helps volatile fumes build up, raising health risks during the next inventory check. My lab always kept a small exhaust fan running in storage spaces. A whiff that something’s “off” signals a quick audit. Even a slight solvent smell means reviewing seals and maybe moving bottles to a fume hood.
Safety stops with the logbook. Details about batch numbers, purchase dates, and expiry keep the risk down. I’ve seen shelf lives ignored, leading to breakdowns in product quality or safety. Every few months, a close look at labels, expiration dates, and the color or texture inside each bottle gives peace of mind. Anything compromised by time or exposure gets quarantined right away.
A locked cabinet does more than keep curious hands out—it saves lives. Only trained staff handle these chemicals, and anyone with access reads the SDS before lifting a bottle. Spelling out protocols isn’t bureaucracy; it’s a sign the organization cares about its workers and values the research.
Investing in better storage—ventilated cabinets, digital logs, sealed containers—pays off every day. Inspection routines don’t just meet regulations; they protect health, data, and investment. Stories from veterans remind young scientists that smart storage isn’t about following rules blindly. It’s about taking ownership, avoiding messes, and building a place where science and safety go hand in hand.
Most folks outside of a chemistry lab probably haven’t heard the mouthful of a name, 2-(2,5-Difluorophenyl)Pyrrolidine. This compound appears in research—and across discussions in chemical safety—thanks to its unique structure and possible use for creating other chemicals. Sometimes, the unknowns are as important as what we already know. Chemicals like these raise eyebrows not just for scientists, but also for people who care about the work environment and the impact these substances have beyond the lab.
I’ve worked with enough specialty chemicals to have seen people underestimate small molecules, thinking harmful effects only come from what’s already regulated. That’s not the case. A compound sporting two fluorine atoms rings alarm bells: fluorinated substances often resist breaking down and can stick around in the environment or body longer than intended. Peer-reviewed research on the exact toxicology of 2-(2,5-Difluorophenyl)Pyrrolidine stays thin, but experience with similar chemicals—often those with aromatic rings and halogens—shows they can sometimes be stubbornly persistent, tricky for our bodies to process, and have unpredictable health effects.
Anyone who’s breathed in odd smells or felt a sting from a chemical splash knows risk isn’t just theoretical. Workers may handle this compound, sometimes with not enough background about what even fleeting skin contact or a single inhalation does in the long run. This sort of compound isn’t something to brush off. Existing chemical databases hint that related molecules could irritate eyes, skin, or lungs, or end up as bigger health headaches over repeated exposure. Chronic exposure to some pyrrolidine derivatives causes problems in animals—think liver issues, changes in blood chemistry, sometimes even disruption to the nervous system.
Doctors and safety experts want solid answers. With a chemical like this, finding out exactly how much is dangerous takes time. Usually, hazard information develops through animal testing, computational modeling, and sometimes bitter lessons from accidental exposure. The lack of full toxicology data isn’t a green light for carelessness. Out of caution, experts suggest gloves and goggles as a rule, even if the safety sheet lacks specific warnings. Any spills get contained right away. Work in a fume hood, far from snacks and drinks.
A healthy respect for new and partially-studied chemicals grows with each batch handled. Moms, teachers, and regulators all want to lower risk. Good practice in the lab means pressuring suppliers for more safety data, logging incidents, and using less hazardous alternatives if available. It matters at every step—from production to disposal. Avoiding complacency isn’t just following the rules; it’s about keeping the people mixing, measuring, and moving these chemicals out of harm’s way. Every detail counts.
Chemicals from the pyrrolidine family and their fluorinated cousins spark urgent questions: what cleaner alternatives exist? What investment in research will tell us quickly if persistence or toxicity becomes a threat? The community that handles these compounds—scientists, environmental workers, industry leaders—should push for full screening before large-scale use. Tech and testing capabilities grow year after year. Facts, transparency, and teamwork give us a shot at a safer workplace and cleaner world.
Purchasing a specialty chemical such as 2-(2,5-Difluorophenyl)Pyrrolidine demands attention to quality details. I’ve seen what happens in research environments when purity slips—even minor contamination can derail an entire project or cause inconsistent results down the line. Unwanted byproducts, water content, or trace metals have the potential to throw off a reaction or create safety concerns. Labs pay for certainty. Knowing exactly what’s in a vial provides more confidence than a technical data sheet ever could.
For this type of fluorinated pyrrolidine, the most accessible grades usually sit at 95% to 98% purity, classified as “technical” or “laboratory” grade. Analytical data from major suppliers tends to point to these thresholds as their guaranteed minimums, with some high-spec batches reaching 99% or just above. See the chromatograms and NMR spectra: reputable vendors lay them out to show off those tiny impurity peaks left behind. This is more than just good form—it reflects the reality that pharmaceutical, agrochemical, and advanced materials research rarely accept a chemical below 95% without extra cleaning up.
The route to higher purity starts with the synthesis. Methods relying on clean reagents, controlled temperature, and vigilant chromatographic separation offer better chances at hitting 98% or more. I’ve talked with synthetic chemists who insist that even a slight tweak—say, swapping a solvent or changing the purification media—introduces byproducts. These might not be an issue for small-scale screening, but in scale-up or regulatory development, that trace impurity may become a stumbling block. We’re not just talking about aesthetics—in the pharmaceutical world, traces of an unknown signal make regulatory filings drag on for months.
As much as certificates of analysis inspire trust, nothing beats third-party confirmation. Over the years, I’ve watched research groups run their own NMR and HPLC on every new batch, even from trusted names. A vendor might claim 98%, but actual spectra paint the real picture. Getting what you pay for takes more than checking off a data sheet; it means asking for batch-specific documentation, then verifying on your own equipment.
Grades higher than 98% sometimes appear labeled as “high-purity” or “pharmaceutical grade.” That doesn’t always match up with formal regulatory statuses, but it signals extra care in handling, packaging, and analysis. Still, supply chain factors make ultra-pure batches less common: cost runs high, lead times stretch out, and material moves in smaller lots. I’ve seen researchers hit project delays waiting for a specific grade to clear customs inspection because of just these supply chain challenges.
No single batch ever tells the full story. It’s easy to reach for the highest labeled purity, yet cost and urgency push researchers to balance risk and reward. If a synthesis tolerates minor impurities, then technical grade can make sense for proof-of-concept work. For anything heading into downstream processing or scale-up, documented high grade backed by recent analytical data should stand as non-negotiable. Storing such sensitive compounds correctly—dry, cool, away from bright light—also helps defend against purity losses. Too many times I’ve returned to a vial, only to find new peaks on the chromatogram from plain old exposure to humidity or careless handling.
Transparency from suppliers should be standard practice. Detailed analytics, clear batch histories, and rapid response to customer questions cut down on wasted time and resources. Researchers too, have a responsibility to question and verify. Testing, not just trusting, keeps projects on track, budgets under control, and helps ensure safety at every step. Chemical purity may sometimes seem like a numbers game, but in day-to-day work, it’s a foundation for progress.
| Names | |
| Preferred IUPAC name | 2-(2,5-difluorophenyl)pyrrolidine |
| Other names |
2,5-Difluoro-2-phenylpyrrolidine 2-(2,5-Difluorophenyl)pyrrolidine |
| Pronunciation | /ˈtuː ˈtuː ˌfaɪv daɪˈfluːrəˈfiːnɪl pɪˈrɒləˌdiːn/ |
| Identifiers | |
| CAS Number | 1197953-73-1 |
| Beilstein Reference | 2518737 |
| ChEBI | CHEBI:189306 |
| ChEMBL | CHEMBL429047 |
| ChemSpider | 16252513 |
| DrugBank | DB08240 |
| ECHA InfoCard | 03a1cfce-d2c1-4506-9f8f-9a35b87fc0ed |
| EC Number | 3.2.1.20 |
| Gmelin Reference | 84177 |
| KEGG | C19826 |
| MeSH | C21:Chemicals and Drugs; D02:Organic Chemicals; D03:Heterocyclic Compounds; D04:Cycloparaffins; D05:Pyrrolidines |
| PubChem CID | 137319097 |
| RTECS number | SK3000000 |
| UNII | V939401ILA |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DTXSID7026299 |
| Properties | |
| Chemical formula | C10H11F2N |
| Molar mass | 171.18 g/mol |
| Appearance | Light yellow liquid |
| Odor | Odorless |
| Density | 1.23 g/cm3 |
| Solubility in water | Insoluble |
| log P | 2.4 |
| Vapor pressure | 5.7E-2 mmHg at 25°C |
| Acidity (pKa) | pKa ≈ 11.2 |
| Basicity (pKb) | pKb = 3.38 |
| Refractive index (nD) | 1.540 |
| Dipole moment | 2.55 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 337.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -247.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3934.9 kJ/mol |
| Pharmacology | |
| ATC code | N05CM24 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS07 |
| Pictograms | `COC(=O)C1=CC=CC=C1N2CCCC2` |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P264, P280, P302+P352, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 81.4±25.7 °C |
| LD50 (median dose) | LD50: Oral Rat >2000 mg/kg |
| PEL (Permissible) | Not established |
| REL (Recommended) | 50 mg |
| Related compounds | |
| Related compounds |
2-(2,4-Difluorophenyl)pyrrolidine 2-(3,5-Difluorophenyl)pyrrolidine 2-(2,5-Dichlorophenyl)pyrrolidine 2-(2,5-Dimethylphenyl)pyrrolidine 2-(2,5-Difluorophenyl)morpholine |