Long before chemists nailed down 2,2,4,4,6,8,8-Heptamethylnonane as a product of synthetic muscle, the search for highly branched hydrocarbons grouped together experts from fuel engineering, organic chemistry, and industrial process design. Petroleum refining in the 20th century shaped the path for such molecules, as fuel designers needed precise blends to boost octane ratings without encouraging knock in modern engines. This compound, recognized for its intricate structure, came to represent many improvements achieved through isomerization and alkylation processes. Looking back, I see research efforts reflected in today’s broad industrial toolkit: techniques for precise molecular tailoring did not develop overnight. They grew out of decades of incremental advances and dogged laboratory troubleshooting.
At its core, 2,2,4,4,6,8,8-Heptamethylnonane embodies a highly-branched alkane, packing nine carbons into a shape that reminds chemists why carbon’s versatility has powered the industrial world. Its greatest utility shows up where a low freezing point, high boiling range, and minimal reactivity with metals or plastics makes sense—like in high-performance fuels and specialized reference fluids. Chemical manufacturers list it under various names and supply it in bulk for laboratories, research groups, and process engineers who want to test materials, calibrate instruments, or design new products.
2,2,4,4,6,8,8-Heptamethylnonane appears as a clear, colorless liquid with a distinctively mild, hydrocarbon-like scent. Its high branching cuts down on intermolecular forces, which means a lower melting point and higher resistance to volatilization compared to more linear alkanes. The molecular weight lands at 242.5 grams per mole. Its boiling point sits around 193–197°C, while the melting point hovers well below room temperature, making it a stable liquid under most conditions. Water solubility remains negligible, reflecting its hydrophobic, nonpolar nature. The compound exhibits exceptionally low reactivity due to the saturation of all carbon atoms, avoiding oxidation and photodegradation under regular laboratory lighting. Flammability cannot be ignored, as with any hydrocarbon, and safety precautions always belong at the forefront when heating or transferring this substance.
Industrial and chemical suppliers specify this compound by purity levels, usually more than 98%, sometimes as high as 99.5% for analytical applications. Labels must reference the CAS number (13752-43-9) and include batch information to allow for traceability in case of any anomaly during use. Drums and containers list both the chemical and any hazards according to GHS, emphasizing flammability, proper storage, and incompatibility with oxidants. Analytical certificates should verify absence of key impurities, especially sulfur or oxygen-containing contaminants, which can interfere with sensitive instrumentation. I have personally seen labs get tripped up by off-spec reference fluids where such details were overlooked; the lesson remains clear: always check documentation, even for routine testing.
Commercial production of 2,2,4,4,6,8,8-Heptamethylnonane leans on classic organic chemistry. Alkylation of isobutene with higher alkanes, commonly catalyzed by Lewis acids, builds out the branched skeleton. Fractional distillation follows, separating the desired product from similar side-chain isomers. The process rewards precision and steady temperature control, since impurities frequently complicate distillation if thermal budgets slip. Over time, process engineers refined yields and reduced byproduct waste by modifying catalysts and optimizing reaction conditions.
This alkane’s remarkable inertness draws attention in both academic and applied chemistry. It shrugs off typical oxidizing and reducing agents under normal conditions. In fact, that characteristic lands it among a select few standards for engine testing—its resistance to reaction stabilizes test results. That is not to say it cannot react at all: when exposed to intense chlorination, it will produce multiple chloro and polychloro derivatives, though conditions must be harsh: high temperature, UV irradiation, or a radical initiator. In practice, few labs go down this route except when probing combustion pathways or looking for trace combustion products in engines or reactors.
You might spot this alkane listed as HMN, Heptamethylnonane, or even by trade names tied to specialty reference fuels. Commercial catalogs track synonyms, and research articles sometimes abbreviate it to "iso-nonane" for convenience, though purists frown on the imprecision. CAS 13752-43-9 anchors its identity in regulatory and safety documentation. Some technical papers describe it as a reference component in the ASTM research octane number blends or as a calibration standard in VOC emission testing. For buyers and users, this wide set of synonyms means careful review, so an order matches project needs.
As hydrocarbons go, 2,2,4,4,6,8,8-Heptamethylnonane keeps a low chemical profile. Safety Data Sheets (SDS) advise flammable liquid protocols, grounded containers, and minimized spark sources during transfer or handling. Labs storing significant amounts need explosion-proof ventilation. Spills, though rare in careful operations, require immediate cleanup with non-sparking tools and absorbent, followed by proper disposal in compliance with local environmental guidelines. Operators handling this chemical in pilot-scale or production facilities wear flame-resistant lab coats, goggles, and gloves. Fire departments brief themselves on its burning characteristics—low smoke, high-energy flame, with potential for vapor travel along surfaces.
Few compounds carve out such a specific identity in reference and calibration work. 2,2,4,4,6,8,8-Heptamethylnonane acts as a key ingredient in the formulation of research octane number (RON) and motor octane number (MON) standard fuels for engine testing: its stable burning, low side reactions, and well-characterized nature made it a mainstay in fuel research. Environmental laboratories blend it into VOC emission simulants, while instrument makers use it for chromatograph calibration. If you look at fuel performance studies—especially those investigating anti-knock additives—this molecule often sits at the heart of engine bench tests. In aerospace and automotive labs, it supports lubricants and synthetic fuel blends. Outside strictly technical fields, its extremely non-polar structure lets separation scientists use it to test and validate stationary phase selectivity in advanced chromatography columns.
Scientific output on 2,2,4,4,6,8,8-Heptamethylnonane might not fill journals, but researchers lean on it as a trusted baseline for experiments and instrument calibration. Combustion chemists break down its reaction intermediates under controlled flame conditions to map out hydrocarbon breakdown pathways. Process engineers study its performance as a blending agent, evaluating whether upgrades in refinery processes or additive packages shift ignition resistance, burn rate, or vaporization. I find its role as a validation compound under-appreciated, especially given how much modern analytical chemistry looks for robust standards that don’t shift or degrade over repeated cycles.
Studies completed by occupational health bodies and chemical safety assessors highlight low acute toxicity for 2,2,4,4,6,8,8-Heptamethylnonane, provided exposures remain within typical laboratory or industrial levels. As with most alkanes, ingestion or inhalation of significant vapor quantities can suppress the nervous system and irritate respiratory tissues, but routine lab handling with proper extraction and personal protection sees little risk of long-term effects. Chronic exposure assessments remain less developed. Given the chemical’s low solubility in water and high volatility, spills or leaks present environmental challenges mostly in air quality and fire hazards, rather than persistent bioaccumulation. Researchers and occupational hygienists have pressed for more environmental fate and aquatic toxicity studies, since highly branched alkanes occasionally resist breakdown by standard microbial populations—something I encountered during a water quality survey in refinery outflows.
Shifts in the energy landscape, especially moves toward sustainable fuels and stricter emission standards, mean reference hydrocarbons like 2,2,4,4,6,8,8-Heptamethylnonane still carry real research weight. As automotive sectors transition toward low-carbon and bio-based alternatives, standardized engine testing demands stable, reproducible fuel surrogates. Next-generation chromatographic and analytical method development calls for stable, clearly defined standards, and this molecule fits that bill better than most. Ambitious process chemistry groups have started looking at improving synthetic pathways, either to boost yields or reduce the ecological footprint of production, especially by moving away from heavy-metal catalysts. Its extended family of highly branched hydrocarbons continues to stand as benchmarks for combustion efficiency, volatility, and compatibility studies, so ongoing research and technical scrutiny guarantee a place for this molecule as a reference point for years to come.
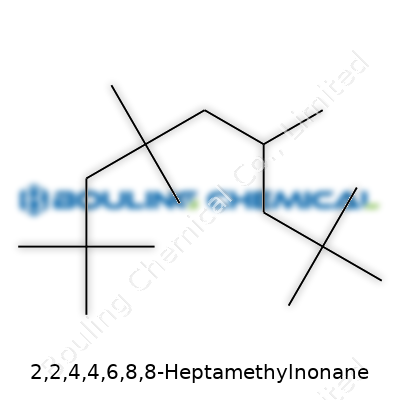
People who work with chemistry often talk about structures, chains, rings, and formulas. But let’s slow down and look at a specific molecule—2,2,4,4,6,8,8-Heptamethylnonane. Its name might trip up the tongue, yet the thinking that goes into figuring out its chemical formula opens a window into the practical side of science. This compound is a highly branched alkane, not something usually mixed into household products or common industrial feedstocks. Its skeleton holds meaning for chemists looking for patterns, properties, and possibilities.
Decoding the formula starts with looking at the nonane backbone, that’s nine carbon atoms in a row. Now, add a methyl group—one carbon, three hydrogens—at the second, fourth, sixth, and twice at the eighth and again at the second and fourth. That takes a simple straight chain and fills it with seven extra side branches. In terms of formula, pair up the carbons and hydrogens, and you get C16H34.
Nobody spends time on a compound like this just for the thrill of reciting a tongue-twister name. Its real-world value shows up in fuel testing, engine research, and the study of how structure changes performance. Highly branched alkanes like 2,2,4,4,6,8,8-Heptamethylnonane resist knocking in engines, making them key in developing high-octane fuels. Gathering this knowledge has led to better engines and fuel mixtures, which help reduce waste and boost efficiency in transportation.
Looking at isomers, organic chemists can predict how slight structural tweaks bring surprisingly different boiling points, melting behavior, and stability—even if the chemical formula matches that of other molecules. Physical labs measure these differences to inform decisions about refinery processes, additives, and climate impact. Research into chemicals like this translates directly into smarter fuel formulations.
Learning to draw and analyze such branched molecules comes early in university chemistry labs, where it confuses many. Teachers work harder each year to bridge the gap for students. Handheld models, computer simulations, and interactive examples make a difference. The world faces a shortage of chemical expertise in some regions, so building comfortable relationships with structure and formula early, using clear examples, should get more support across schools and universities.
Digital software like ChemDraw and the explosion of open-access databases now put these molecules at the fingertips of anyone willing to learn. Still, nothing replaces pencil-on-paper practice and occasional mistakes—those moments that stick as “aha!” memories.
Fuel innovation, green chemistry, and safer product design all trace back to knowing what’s in a molecule. Governments and school boards can support curiosity in chemistry with stronger funding for lab experiences and practical training. Collaborations between industry and education help, too: industry chemists returning to classrooms, or students getting to tour real labs, grow problem-solving skills that textbooks struggle to ignite.
The formula C16H34 attached to 2,2,4,4,6,8,8-heptamethylnonane stands for much more than a dry set of numbers and letters. It marks a place where careful study, thoughtful design, and hands-on practice turn chemistry from a subject into a skill you can use to improve everyday life.
2,2,4,4,6,8,8-Heptamethylnonane started catching my attention years ago during an internship at an industrial lab. We worked with it as a reference compound in octane rating tests, long before cleaner fuels took over headlines. Folks in quality control rely on it to help benchmark combustion in gasoline, specifically in the ASTM Research and Motor Methods. This hydrocarbon offers clear advantages as a standard because it resists knocking better than most, allowing refineries to fine-tune fuel blends with repeatable test results. Research Octane Number (RON) matters for engine efficiency, and blending away from standard values risks engine wear or wasted fuel. Test labs keep bottles of it on shelves for this reason, not just for data points but for keeping engines running smoothly across continents.
In organic chemistry, sometimes you need a solvent that stays out of the reaction—one that neither grabs onto ions nor interferes with catalysts. 2,2,4,4,6,8,8-Heptamethylnonane does just that. It serves as a non-polar medium for dissolving stubborn non-polar compounds. Colleagues in the coatings industry often mention it for specialty paints and adhesives since its chemical structure won’t attack pigments or binders. That’s a detail many overlook until color stability goes off-target halfway through a batch run.
Anyone who has cracked open a technical data sheet on high-end lubricants for aerospace or automotive work has likely spotted variants of branched hydrocarbons. This compound flows easily at both low and high temperatures, so it finds a place in synthetic lube formulations. Engines, turbines, and gears last longer and require less upkeep when lubricants don’t leave gummy residues or break down under pressure. Stories of gearboxes seizing up because of breakdown-prone base oils aren’t just workshop horror tales—they’re real, and costly. Using sturdy molecules like this sidesteps those issues, especially in applications facing wide temperature swings or long service cycles.
A well-stocked metrology lab banks on accuracy. Scientists rely on 2,2,4,4,6,8,8-Heptamethylnonane when calibrating chromatographs or mass spectrometers used in hydrocarbon analysis. You can’t separate petroleum components or environmental samples without correct calibration. Lab technicians depend on its well-characterized retention time and fragmentation pattern to check if equipment is drifting or samples are contaminated. Missed calibrations lead to false readings, product recalls, or overlooked spills—problems that stack up in the real world, far away from textbook labs.
Working with volatile organics like this one always circles back to safety. Chemical exposure isn’t an abstract hazard. Respiratory protection, proper ventilation, and careful transfer protocols matter, both at the plant and in the lab. Regulations around storage and disposal line up for a reason—fires, air releases, and health problems travel fast when safety steps get skipped. I’ve seen colleagues become complacent with routine substances. But there’s never an excuse for skipping risk assessments or personal protection just because a chemical seems familiar.
As tight regulations and alternative fuels pick up pace, some uses of specialty hydrocarbons are bound to shift. Still, the demand for controlled reference materials, industrial solvents, and robust lubricants won’t vanish overnight. Adapting safer practices and keeping an eye on alternative compounds with lower environmental impact offers a path that supports innovation as well as public health. That mix—practical use, safety, and future focus—gives a well-known compound like 2,2,4,4,6,8,8-Heptamethylnonane staying power in science and industry.
This chemical looks like a tongue-twister, but 2,2,4,4,6,8,8-Heptamethylnonane shows up in industries tied to fuels and solvents. Some folks know it as HMN or ‘isododecane,’ and it crops up where there’s a need for specialty hydrocarbons. Refineries sometimes use compounds like this when researching new gasoline recipes or when testing emission control systems. You might catch a whiff of it in product labs, not in grocery stores or homes.
Breathing in 2,2,4,4,6,8,8-Heptamethylnonane at work – say, in a lab or at a plant – leads to one main concern: exposure. It’s a hydrocarbon, so compare it to sniffing gasoline vapor. Short-term exposure usually brings dizziness, headaches, or irritation in the eyes or throat, like many other volatile organic compounds. I remember a friend in an industrial lab complaining about headaches during a spill cleanup, even though he wore basic personal protective equipment. Most acute symptoms faded after fresh air and some time away from the site.
Long-term effects haven’t been studied in many people. Animal testing reports show respiratory tract irritation when animals breathe high concentrations. No reputable scientific group has classified it as a carcinogen or listed major reproductive risks, at least not at the levels most workers stumble into during routine handling. This isn’t to say it’s entirely safe, just that—like many petroleum-based solvents—the bigger threats sit with continuous, high-level exposure or sloppy handling.
Most mishaps come down to spills, poor ventilation, or noxious fumes sneaking past personal protective gear. Strict industrial hygiene usually keeps issues small, since most labs and refineries rely on good hoods, gloves, and air monitors. If these drop out of the equation, symptoms show up faster. In a poorly ventilated shop, heavy fumes could lead to confusion or even loss of consciousness, especially if someone’s working alone.
The chemical burns pretty well too, which means the greater risk to workers during bigger leaks comes from fire and explosion, not chronic toxicity. That’s a lesson drawn from petroleum safety drills—ignition risks often outstrip slow-and-steady exposure worries, especially where flammable vapors gather near sparks or heat.
Any crew working with HMN needs strong ventilation, reliable respiratory masks, gloves that seal out solvent, and spot-on training. Health monitoring comes into play if time in contact with vapors stretches long. I’ve seen workplace safety reports stress spill response kits in any room holding this chemical, so secondary exposure gets cut short fast.
For the curious at home, 2,2,4,4,6,8,8-Heptamethylnonane usually doesn’t show up in household goods, so average folks won’t cross paths with it outside industrial settings. Most health and safety research steers toward safer substitutes if large-scale, routine exposure seems unavoidable.
Regulators want clear safety data sheets and proper labeling, so everyone knows what they’re working with and how to protect themselves. The push to cut airborne volatile solvents from factories and labs continues, leading companies to manage risks and sub in less hazardous options as research brings them into view. Considering HMN’s flammability and acute effects, simple steps—staying alert, knowing the risks, and using good ventilation—pay off if you find yourself on the job around this chemical.
Working with 2,2,4,4,6,8,8-Heptamethylnonane in any lab or industrial setting goes beyond checking off safety boxes. This hydrocarbon might seem unassuming, but anyone who's opened a container of volatile organics knows the headaches that come from skipping the basics. It's clear that this compound, like many branched alkanes, holds a low flash point and gives off fumes you don't want to breathe in for long.
Once, I watched a technician carry out a transfer in a cramped storeroom without turning on the extractor fan. Within minutes, the smell took over, eyes watered, and conversation stopped. This stuff doesn’t care about your schedule—it will fill a space quickly, so wide-open airflow and proper fume hoods become the backbone of handling. For context, the flash point sits squarely in the range where a small spark can turn a careless transfer into something more dramatic and far more expensive.
There's also the matter of ignition sources. Phones left charging, badly maintained electrical panels, even metal tools scraped across a concrete floor can provide a spot of trouble. A grounded, dedicated area—marked and clear—is worth every minute you spend setting it up. Fire extinguishers rated for flammable solvents shouldn’t hide behind locked doors. Keep sand buckets nearby as a fallback for spill containment.
Too many accidents start with flimsy or reused containers. This alkane eats through some plastics or slowly perishes rubber seals. Thick-walled, seal-tight metal drums or certified glass bottles are best. I always label everything with bold, chemical-resistant ink. No label, no use, even if I recognize the smell. That simple rule has saved me and my colleagues more than once from surprise exposures.
Many overlook compatibility charts. Not every storage shelf fits the bill for long-term chemical storage. Metal reacts with time; gaskets degrade. Regular checkups keep the sneaky leaks and vapor escapes in check. A drip pan underneath adds another layer of security, catching small leaks before they start a chain reaction with other nearby chemicals.
In my experience, the best equipment in the world can’t fix carelessness or inexperience. Everyone on shift benefits from short, direct training sessions that actually cover the quirks of the materials in use. Dragging out the Safety Data Sheet over coffee, walking through real spill drills, and making eye protection and gloves a habit—these steps beat reading bullet points off a screen. Emergencies rarely announce themselves ahead of time, and practice means muscle memory kicks in rather than panic.
When it’s time to dispose of leftover material, local regulations set the rules. Where I’ve worked, the specialist waste contractor only comes every other week, so planning storage space for waste is a must. Never dump solvents—ever. Double-check drums and get disposal logs signed off for every batch that leaves. It avoids hefty fines and headaches, and it shows respect for the community outside the lab.
At the end of every shift, a few minutes to wipe down benches, close up containers, and update inventory save hours of trouble down the line. Sure, it’s routine. But these tiny actions hold big outcomes. Accidents rarely result from one big mistake—they tend to build up from a handful of small shortcuts.
Anyone storing or working with 2,2,4,4,6,8,8-Heptamethylnonane can protect their health, keep the workspace productive, and prevent ugly surprises by sticking to practical habits and insisting on good training. The right approach makes safety feel natural, not forced.
Some compounds aren't part of the everyday shopping list, and 2,2,4,4,6,8,8-Heptamethylnonane fits that category. Organic chemistry labs and niche industrial players use chemicals like this as part of complex processes—fuel research, solvent development, or advanced material manufacturing, for example. It's rare to hear about someone needing this liquid for home use, so right away, distribution gets limited to specialized suppliers.
Most regular folks will never see bottles of this stuff on a store shelf. Instead, you find it within the catalogs of chemical suppliers that operate under heavy rules: Sigma-Aldrich, Thermo Fisher, TCI, and so forth. These suppliers operate with strict paperwork and verification. Orders for a molecule like 2,2,4,4,6,8-Heptamethylnonane need proof—of proper licensing, a business or research address, and legitimate intent. A home address without credentials gets the order flagged or canceled.
For me, early in my research career, chemical purchasing worked like clockwork only when the department’s purchasing manager stepped in. She checked each item against safety records, disposal plans, and legal guidelines. Turns out, compliance is king—one missed training session, or one vague project description, and the order sat in limbo for weeks. There’s technical value in this added friction, though. Keeping hazardous substances out of untrained hands keeps both the workplace and the wider public safer.
This market isn’t about selling to the highest or most eager bidder. Expertise and transparency matter—every supplier profile I’ve dealt with asked for both. Clear project descriptions, signed agreements, and safety protocols come as prerequisites, not afterthoughts. People managing chemical supplies have learned hard lessons from past accidents and misuse.
Some groups try to skirt these restrictions by sourcing chemicals from overseas sellers on e-marketplaces. I wouldn’t trust that path. Shipments can get seized, mislabeled, or contaminated. Respected suppliers run analytic checks—infrared spectra, quality certificates—proving that their barrels match what’s on the label. Off-the-books sellers shortcut testing and put buyers at risk.
Better safety isn’t just about locking supply chains. Suppliers should put clear, plain-language guides right there with their products—hazard pictograms, storage tips, and up-to-date local law references. Many researchers—especially newcomers—learn the hard way about what can go wrong with a mislabeled flask. Top suppliers like Sigma-Aldrich have started rolling out quick-access digital safety sheets, which helps a lot. There’s still room to grow, though. More countries could pitch in with digital permitting processes and centralized supplier registries so buyers get one-stop verification, not paperwork whiplash.
Chemistry continues opening worlds, but only if buyers and sellers respect the boundaries set by ethics, law, and science. Shopping for 2,2,4,4,6,8-Heptamethylnonane means walking that line, trusting tried-and-true suppliers, and growing a culture where safety never becomes an afterthought. Honest guidance and watchdog systems steer the ship—and every responsible purchase choice nudges the market toward stronger practices.
| Names | |
| Preferred IUPAC name | 2,2,4,4,6,8,8-Heptamethylnonane |
| Other names |
Isooctyl isopentyl methane Heptamethylnonane |
| Pronunciation | /ˌhɛp.təˌmɛθ.əlˈnəʊ.neɪn/ |
| Identifiers | |
| CAS Number | 17301-94-9 |
| Beilstein Reference | 1871647 |
| ChEBI | CHEBI:89070 |
| ChEMBL | CHEMBL164460 |
| ChemSpider | 12319783 |
| DrugBank | DB16673 |
| ECHA InfoCard | 100.116.403 |
| EC Number | 92045-48-8 |
| Gmelin Reference | 60752 |
| KEGG | C09045 |
| MeSH | D058728 |
| PubChem CID | 117656 |
| RTECS number | SY8475000 |
| UNII | K3M09SLM3O |
| UN number | UN1993 |
| CompTox Dashboard (EPA) | DTXSID0030442 |
| Properties | |
| Chemical formula | C16H34 |
| Molar mass | 272.55 g/mol |
| Appearance | Colorless liquid |
| Odor | Odorless |
| Density | 0.759 g/mL at 25 °C (lit.) |
| Solubility in water | Insoluble in water |
| log P | 6.18 |
| Vapor pressure | 0.334 mmHg (25°C) |
| Acidity (pKa) | The acidity (pKa) of 2,2,4,4,6,8,8-Heptamethylnonane is approximately >50. |
| Magnetic susceptibility (χ) | Diamagnetic |
| Refractive index (nD) | 1.422 |
| Viscosity | 1.13 cP (25 °C) |
| Dipole moment | 0.00 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 416.8 J·K⁻¹·mol⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -352.7 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | –8087.7 kJ/mol |
| Pharmacology | |
| ATC code | No ATC code |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | ["GHS07"] |
| Signal word | Warning |
| Hazard statements | H411: Toxic to aquatic life with long lasting effects. |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P271, P273, P301+P310, P303+P361+P353, P331, P370+P378, P403+P235 |
| NFPA 704 (fire diamond) | NFPA 704: "1-3-0 |
| Flash point | 67 °C (closed cup) |
| Autoignition temperature | 220 °C (428 °F; 493 K) |
| Lethal dose or concentration | LD₅₀ (oral, rat): >5000 mg/kg |
| LD50 (median dose) | LD50 (median dose) of 2,2,4,4,6,8,8-Heptamethylnonane: >5 g/kg (rat, oral) |
| NIOSH | RN 4396 |
| REL (Recommended) | 10 mg |
| IDLH (Immediate danger) | There is no IDLH value established for 2,2,4,4,6,8,8-Heptamethylnonane. |
| Related compounds | |
| Related compounds |
2,2,4-Trimethylpentane Isooctane 2,2,3,3-Tetramethylbutane Triptene |