Curiosity drives chemists to poke at the unknown, to pull threads from the tangled web of molecules. 1H-Pyrrole, discovered during the fevered years of the 19th century, pulled its weight early on in the labs of European chemists who tried to scrape order out of chaos. The first traces of pyrrole came from dry distillation of animal matter—a routine born from curiosity and a bit of stubbornness. Over the years, stronger methods replaced the crude origins, but the same drive remains. Pyrrole’s aroma, some say, hints at roasted coffee, and it nudged its way into focus because small molecules, given the right attention, prove themselves essential. Pyrrole sparked entire categories of research in organic chemistry, especially in the race to understand what makes pigments, vitamins, and pharmaceuticals tick.
1H-Pyrrole, often just called pyrrole, is a five-membered aromatic ring holding a nitrogen atom in its embrace. Industry and academia both borrow this simple molecule for building more complex structures—whether that's in the pigment industry, pharmaceutical development, or as starting material for famous compounds like porphyrins and heme cofactors. I’ve run across it countless times during my own research, usually when a stubborn synthesis requires a bit of aromatic backbone. Most bottles you’ll find in the lab are clear, labeled with warning stickers, and somehow, over the years, it earned a spot in the core shelf alongside other building blocks.
Set a bottle of pyrrole on the bench and you’ll notice a colorless to pale yellow liquid with a peculiar smell—more on the sharp side. With a boiling point near 130°C and a melting point around -24°C, pyrrole remains a liquid across a range of lab temperatures, making it easy to handle. It mixes with many organic solvents but shies away from water. Chemically, pyrrole’s aromatic ring stands out. Unlike benzene, the nitrogen atom tugs at electrons, making the ring less rich but lending unique reactivity, especially in electrophilic substitutions. The nitrogen can participate in hydrogen bonding, giving the molecule a versatility that synthetic chemists covet. It resists strong bases but doesn’t stand up to acids quite so well; protonation can disrupt its aromaticity, making it sensitive to harsh handling.
Lab bottles arrive stamped with the familiar C4H5N formula, CAS registry number 109-97-7, and a purity rating that chemists glance at before using. Standard containers often weigh between 100 to 500 grams, sealed tight to keep out light and air. Labels flag the flammability and health hazards—pyrrole doesn’t mess around. Safety data sheets lie nearby, spelling out the need for gloves and goggles. Some suppliers go further, providing UV-Vis and NMR spectra along with reagents, catering to users who chase after purity.
Early chemists distilled pyrrole from bones—tough work with smelly consequences. Now, labs favor more controlled synthetic routes. The Paal-Knorr synthesis stands as the old reliable, condensing 1,4-dicarbonyl compounds with ammonia or primary amines. Other approaches rely on dehydrogenating pyrrolines or even reducing pyrrole derivatives for a cleaner yield. I’ve prepared it from furfural and ammonia, a process that impressed me with its simplicity and the stubborn smell that lingers even after scrubbing the glassware. These days, many just order it from suppliers: efficiency often wins.
Pyrrole’s chemistry keeps the attention of synthetic chemists. The ring loves to participate in electrophilic aromatic substitution. It reacts rapidly at the C-2 position—more so than benzene—enabling substitutions with halogens, acyl chlorides, or sulfonyl chlorides. This high reactivity helps in building extended frameworks for pharmaceuticals or electronic materials. On the flip side, controlling mono- versus poly-substitution gives beginners headaches. The nitrogen can act as a nucleophile, and with the right tweaking, you can swap hydrogens for bulky groups or functionalize the nitrogen itself. Many studies focus on oxidation, metalation, and building up oligomers or polymers for electronic devices or pigment manufacture.
Look down a catalog and you might see “Pyrrole,” but synonyms like azole, 1-azacyclopentadiene, or pyrrol make the rounds in chemical circles. Trade names sometimes sneak in, especially from non-English suppliers, but most chemists settle on 1H-Pyrrole for clarity. The molecule stands at the root of a family that includes derivative names like N-methylpyrrole or polypyrrole, both of which reshape the basic skeleton for specialized roles.
I’ve known colleagues to overlook just how volatile and irritating pyrrole can be. The liquid’s low flashpoint means open flames spell trouble, and inhaling the vapor sure isn’t pleasant. Work in a fume hood, with gloves and goggles, becomes second nature for anyone handling this compound for more than a day or two. Spills get mopped up fast. Safety standards in most labs demand storage away from oxidizers and acids—these combinations risk fire and toxic byproducts. Disposal takes coordination with hazardous waste outfits, making sure pyrrole never heads down the drain. Documentation and labeling sit high on the checklist, both for legal standards and for the safety of everyone working nearby.
That simple five-membered ring shines in more places than many would guess. Pyrrole forms the backbone of biologically vital compounds—porphyrins, heme, and chlorophyll stand out—not just in textbooks but in drug discovery and veterinary formulations. The pigment industry uses it for ink and dye precursors; its ability to create strong colors feeds into textiles and paints. Polypyrrole, a polymer of this base molecule, stands out for use in batteries, sensors, and even in developing flexible electronics. I’ve seen grant proposals filter across my desk, all hoping to exploit pyrrole derivatives in solar panels or as anti-corrosive coatings. It also supports the synthesis of advanced pharmaceuticals, agrochemicals, and high-performance materials. Its adaptability impresses anyone who has used it for more than a routine synthesis.
Research on pyrrole never settles. Teams chase after new derivatives, exploring how tweaks at the nitrogen or ring positions influence reactivity, stability, or bioactivity. Medicinal chemists hunt for novel pyrroles to treat cancer, infection, or inflammation; every major drug database houses a suite of candidates based on this backbone. Material scientists tinker with polypyrrole blends to improve battery storage or design anti-static coatings. Computational chemists model its reactions and predict new synthetic routes. I’ve watched the number of pyrrole-related publications climb, year after year, as new uses in catalysis, optoelectronics, and nanotechnology emerge from unexpected corners.
Toxicologists keep a wary eye on pyrrole, mostly because of its irritation risks and its break-down products. Breathing in large amounts brings headaches, dizziness, and sometimes nausea—none of which make for pleasant days in the lab. Animal studies trace metabolic breakdown and the formation of active N-oxidation products that may pose organ-specific risks. Chronic exposure studies in rodents point to some risk of liver or kidney effects, so safe handling and limited exposure remain cornerstones. The consensus in the field pushes for minimizing inhalation and skin contact, especially in teaching environments where new users might underestimate the risks. Pyrrole’s structure and reactivity play a role here—it can bind to biological molecules, disrupting systems if care isn’t taken.
Interest in pyrrole shows no sign of fading. The trend toward greener chemistry drives scientists to explore renewable feedstocks for its synthesis, cutting waste and reducing environmental footprints. As electronics push for thinner, lighter, and more flexible devices, polypyrrole and its derivatives hold promise as conductive films or coatings. Pharmacologists look to this humble ring to unlock new therapeutic agents, especially against antibiotic-resistant microbes and in the treatment of central nervous system disorders. Sensors built from pyrrole-based materials appear in environmental monitoring and medical diagnostics, two fields set up for rapid growth. The molecule, once a byproduct of animal waste, keeps carving new territory, shaped by the curiosity and persistence of those who study it.
Pyrrole sounds like something you’d find in a lab with bubbling beakers, but this small five-membered ring shows up in places that affect daily life. Chemists have leaned on 1H-pyrrole because its simple structure opens up so many doors. Before I ever sat in a lecture hall, I’d thumbed through product labels and seen all sorts of strange names. Few people realize how many familiar items owe a debt to this one.
Drug makers look for chemical building blocks that offer reliability and flexibility. Pyrrole fits the bill. Popular drugs for treating blood pressure, inflammation, and even cancer have roots in this seemingly plain molecule. Take atorvastatin, a pill millions swallow to tame cholesterol, or the intricate medicines fighting HIV. Often, a pyrrole ring sits right in the middle, holding the structure together and supporting the drug’s work. Chemists love how easily the ring can be modified too, letting them fine-tune a medicine’s action.
Walk down any electronics aisle and you’re probably surrounded by items using advanced coatings and conductive plastics. The backbone of polypyrrole, a material spun from those same five-atom rings, runs through flexible displays, sensors, and even artificial muscles that flex with electric current. In simple language, polypyrrole lets devices move, bend, or measure things that old-fashioned metals would never handle so gracefully. Companies turn to it when they want something lighter, cheaper, and more adaptable than copper or steel. I once handled a roll of polypyrrole film and felt struck by how thin and sturdy it felt.
Anyone who’s dyed their hair or slipped into a pair of jeans owes a silent nod to chemistry. Pyrrole-derived pigments give lasting color to textiles and plastics. The pigment industry searches for compounds that resist fading from sunlight or washing, and this ring keeps proving its worth. Take the manufacturing of phthalocyanine blue, famous for its stability in outdoor paints. Its core borrows from pyrrole chemistry, and that’s a big reason those bright awnings and signs don’t quickly bleach out. Companies keep chasing molecules that pack more color with less energy, both to save costs and help the environment.
Strong demand creates pressure to produce pyrrole and its offshoots in friendlier, less wasteful ways. Factories keep pumping up volumes for pharmacy, electronics, and colorant needs, but no one wants rivers choked by chemical run-off. Some researchers are switching from old fossil-fuel methods to starting points made from plants. I know a small firm trying out new reactors, aiming to slice energy use by half. Months ago, I sat in on a talk where experts debated using water-based reactions to limit toxic byproducts. The hurdles are real — cost, purity, and old habits all slow the shift. Still, governments and watchdogs push for cleaner practices by offering grants and setting tougher safety rules around spills and exposure.
We rarely notice the fine details in a medicine or the protective coating on a gadget, but molecules like pyrrole keep showing up in the backbone of progress. Keeping production clean, affordable, and steady remains a shared job among scientists, industry, and policymakers. If the past proves anything, it’s that these small chemical rings find fresh applications well beyond the pages of a lab manual.
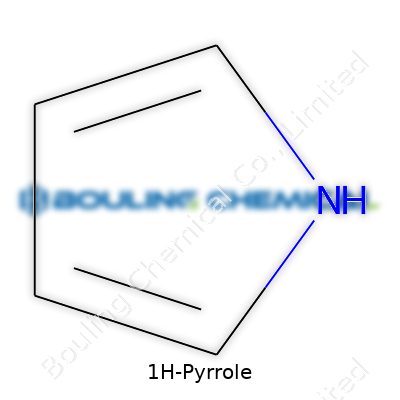
Ask around in any organic chemistry lab and you’ll hear about pyrrole, mostly because it keeps showing up in so many different reactions and compounds. The simple formula—C4H5N—has a way of linking the basics with advanced discoveries in medicine and material science. It looks unassuming, just a five-membered ring with four carbons and a single nitrogen, but this little structure has serious pull in research and industry.
My first time working with heterocycles, I barely noticed pyrrole on the reagent shelf. Yet, take a closer look, and it always finds its place in a lot of crucial drugs, dyes, and even conducting polymers. Hemoglobin and chlorophyll get their green and red magic from porphyrin rings, all built from pyrrole units. It’s the starting point for all sorts of bioactive molecules. Every time you dig through pharmaceutical research, you’ll bump into it—and you can bet that C4H5N backbone is a major player.
Chemistry students sometimes get lost in a sea of complex formulas, but a formula like C4H5N stands out because it’s a reminder: not everything in chemistry is hard to memorize. For a lot of us, seeing that formula on a whiteboard means it’s time to think about aromaticity and electron clouds. Pyrrole as a ring is aromatic, much like benzene, but with nitrogen’s lone pair lending a hand. That opens the door for all sorts of special reactions. It’s why you see it getting tossed in the mix for new antibiotics or next-gen batteries.
In the real world, pyrrole brings up other questions, mostly about production and handling. It smells pretty awful, and it’s not exactly a people-friendly compound if you breathe in the vapor. In my own lab days, we had to use some serious ventilation, and you’d never want to spill it. Large-scale production isn’t without danger, and that’s a big reason folks keep searching for safer methods and the best substitutes for certain reactions. Instead of just dealing with the risks, chemists often tweak the process—choosing milder ways to make pyrrole or using less of it when possible.
Switching to greener synthesis paths is gaining ground now. One promising approach uses safer, bio-based feedstocks. Software-driven analysis helps identify substitutes that do the same job in reactions but give off fewer harmful byproducts—or sometimes, none at all. Investing in proper lab infrastructure—constant airflow, well-sealed storage, and up-to-date training—isn’t glamorous, but it’s necessary. I’ve seen more collaboration across academic and industrial labs in recent years, sharing data on safety and recycling protocols. Cleaner extraction and recycling of pyrrole from industrial waste also lower the impact. It’s not a magic fix, but even small changes really add up.
Listing the chemical formula as C4H5N only scratches the surface. Pyrrole plays a bigger role than its size suggests. For anyone starting out in chemistry or looking for solutions in synthesis, it turns out the most mundane-looking stuff often shapes the future. That’s something no lab manual ever spells out, but it’s what keeps science moving forward—one formula at a time.
Plenty of people haven’t heard about 1H-pyrrole, unless they’re hanging around chemists or dealing with industrial chemicals. It’s got that unmistakable musty, ammonia-like smell, showing up as a colorless liquid. Folks use it for making drugs, plastics, and dyes. Chemistry classes often introduce students to pyrrole’s five-membered ring, but those safety notes are often stuffed near the end of the lab manual, if they're there at all.
I once worked with a team in a lab that kept a dusty bottle of pyrrole tucked away on a shelf. The warning label said “flammable” in thick red print – and that’s no exaggeration. Pyrrole can catch fire quickly and burns with a smoky, stubborn flame. Forget to cap the bottle and the smell will find you fast. Apart from the nose-turning odor, fumes can irritate your eyes, nose, and throat. Take a big whiff or spend a while in a room with a spill, and you’ll know about it; coughing, headache, and dizziness follow close behind.
Bare hands don’t mix well with pyrrole either. Skin won’t melt, but it can sting, leaving redness or a rash. Once, my colleague splashed some and didn’t notice until his skin began itching; he spent the afternoon rinsing and watching for blisters. Ingesting the stuff? Not worth imagining, though animal studies show the liver doesn’t like it one bit.
The paperwork from chemical suppliers and public health agencies isn’t always flashy, but it spells trouble clearly. The U.S. National Library of Medicine lists pyrrole as a chemical that needs respect. In high doses, rats and mice got liver problems, and the lungs didn’t fare much better. Still, you’re unlikely to meet dangerous concentrations outside a laboratory or industrial site. I’ve never seen a story of someone on the street poisoned by it, but folks working with big volumes should pay attention to safety data sheets.
No workplace vet wants to end up needing treatment because their gloves didn't fit right or some fume hood went out. Long-term exposure hasn’t been studied as deeply as with some chemicals, but playing guinea pig with pyrrole isn’t on anyone’s wish list.
Good sense goes a long way with chemicals. Wear gloves that don't leak, don a lab coat, and slap on those goggles. A decent fume hood keeps vapors away from your lungs. Basic first aid like water rinses works wonders if you splash it, but always alert someone if things go wrong. At home, no one should have pyrrole lying about; this chemical belongs in the hands of folks who know what they’re doing.
The real fix sits in training and habits, not just rules on a label. Teams must keep containers tightly sealed, store them somewhere cool, and never let up on the habits that keep a lab running smoothly. Plain talk and regular reminders work better than scare tactics for getting everyone to ditch shortcuts or careless moves.
Pyrrole rarely grabs headlines, but every chemical story is a reminder that substance handling shapes long-term health. Rushing through a chemistry task can hurt — not in some dramatic movie way, but in small, persistent ways that catch up. Regulations around pyrrole set standards, but the real safety happens during each step taken inside a workspace, day after day. Anyone sharing a lab or a plant owes it to the next crew to keep those habits sharp and safety gear clean.
Picture this: you’ve got a bottle of 1H-pyrrole—something that looks like a simple clear liquid, but sneaks up with a strong, sharp smell almost like ammonia and rotten meat. Anyone who’s cracked open a freshly delivered container knows this scent sticks to your mind for hours. I remember my first summer working in a university lab; we joked about “pyrrole perfume,” but no one wanted that stuff anywhere near them for longer than necessary.
Here’s the big picture: pyrrole’s chemistry keeps it as a widely used building block for all sorts of research and industry. Companies making pharmaceuticals, dyes, or specialty plastics count on it. They can’t risk a drop because it breaks down so easily in air and light. It doesn’t simply dry up and go away; it turns dark and sticky, releasing fumes that bring out headaches and complaints from half the building. Breathing in enough vapors sends folks to the safety sheets looking up health warnings.
Nobody loves a spoiled reagent. For 1H-pyrrole, direct sunlight or a warm office shelf proves disastrous. Pyrrole doesn’t just “lose some potency”—it literally changes form, picking up oxygen from the air, gumming up your glassware, and worse, ruining experiments mid-stream. I learned quickly to tuck every bottle deep in a tightly sealed amber container and park it inside the cold storage room—somewhere around 2–8°C, just above freezing. Most labs keep a special cabinet meant for flammables and light-sensitive chemicals, because a bright window or a flickering fluorescent lamp speeds up that breakdown.
Here’s some real talk: Pyrrole catches fire even at room temperature. Spill some, and those vapors fill the air. Spark up an old soldering iron, and you could end up practicing your fire drill for real. Proper chemical storage isn’t just lab paranoia—that’s fire safety in action. If you ask me, every bottle earns a flammable sticker and a home far from anything resembling a heat source or a regular trash bin.
Sure, labeling helps, but the bigger fix starts with training. New lab techs often rush past the boring safety binder, not realizing how quickly a bottle of pyrrole can turn into a headache—or a disaster. Chemists need an attitude of respect, not just vague caution. Easy access to clear instructions helps: think of prominent signs on storage cabinets, quick briefings during lab meetings, and regular reminders of what can go wrong.
Chemical suppliers can lend a hand by providing smaller volume containers so leftovers don’t sit around collecting dust and unpredictable byproducts. Regular checks for leaks or damaged seals make a real difference. Too often, labs let stuff pile up until forced to clean out ancient, half-used bottles. Real safety culture means never letting those bottles reach that point.
One summer, a bottle of pyrrole in the fridge leaked, mixing fumes with perishable samples stored nearby. The cleanup took two full days, with half a dozen people involved—more time than any research deadline can afford. Stories like that reinforce the value of proper storage: better to invest in good habits and right equipment than to pay with wasted time, health scares, or lost money. Treating 1H-pyrrole with respect and storing it smartly spares everyone a world of trouble.
Chemistry rarely hands out simple answers, especially for substances like 1H-pyrrole. Anyone who’s peered into a flask in a college lab or spilled coffee magenta from a research report knows that even small molecules can be stubborn. 1H-pyrrole does things in its own way. On the books, the boiling point sits at about 129 to 131 degrees Celsius. Glance at several reputable sources—you’ll see numbers tightly packed around that mark.
If you handle chemicals for a living, or have a lab hobby after hours, small details make all the difference. Try running a reaction in a closed setup, and watch solvent vanish before your eyes at a temperature you didn’t expect—now you’re pouring money down the drain. I’ve watched students learn this the hard way. A bottle without a clear label, or confusion between 1H-pyrrole and its methylated cousins, quickly adds stress to a process that should run smoothly.
1H-pyrrole gets plenty of attention in pharmaceutical work and materials research. An overlooked boiling point leads to safety issues and product losses. Pyrrole’s boiling point isn’t just a number; it signals how easily it turns into vapor at standard air pressure. Letting it stray too close to that temperature risks turning your neat batch into a puddle of fumes.
In real labs, numbers on a page don’t always match a thermometer in hand. Put 1H-pyrrole on a hotplate without proper oversight, and evaporation sneaks up fast. Permeable lab gloves, leaky containers, and distracted interns can send that unmistakable, sour aroma of pyrrole drifting through the air.
Handling this chemical demands planning. A fume hood is your friend. Small batches lower the chance of big spills. Most scientists I’ve worked with swear by storing pyrrole in cool, dark bottles with tight seals. In the modern world, automation helps as well, with hotplates that switch off at preset limits. None of these tricks matter unless people pay attention to each step and double-check their equipment.
Plenty of newcomers ask why some liquids boil long before water, and others hang on till a stovetop glows orange. The answer—molecular structure shapes everything. Pyrrole has a five-membered ring and a nitrogen atom. Its structure brings weaker attractions among molecules than you’d find in water. So it flies away at a lower temperature. But that ring system, and the lone nitrogen, help give pyrrole its unique smell, chemical reactivity, and value on the market.
Recently, interest in “green” chemistry has thrown light on old solvents like pyrrole. People want methods that keep workers safer and air inside the lab breathable. The drive for safer handling leads to more locked cabinets and better training—even smart sensors that warn of vapors building up. Manufacturers can support lab staff by shipping smaller volume bottles, clear labels, and supplying up-to-date safety sheets with every order.
Getting comfortable with details like boiling points isn’t just a textbook exercise. It keeps labs running well and encourages every chemist to treat each substance with respect. Pyrrole demands careful storage and monitoring, but it opens doors to creative molecular work—provided the basics are not ignored.
| Names | |
| Preferred IUPAC name | Pyrrole |
| Other names |
Azole Pyrrol |
| Pronunciation | /paɪˈroʊl/ |
| Identifiers | |
| CAS Number | 109-97-7 |
| Beilstein Reference | 10405 |
| ChEBI | CHEBI:35547 |
| ChEMBL | CHEMBL1167 |
| ChemSpider | 5549 |
| DrugBank | DB04238 |
| ECHA InfoCard | 100.006.175 |
| EC Number | 201-509-1 |
| Gmelin Reference | 60430 |
| KEGG | C00261 |
| MeSH | D011693 |
| PubChem CID | 794 |
| RTECS number | UY7875000 |
| UNII | 78A24W2X0T |
| UN number | UN2065 |
| CompTox Dashboard (EPA) | DTXSID4022929 |
| Properties | |
| Chemical formula | C4H5N |
| Molar mass | 67.09 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | Aromatic |
| Density | 0.967 g/mL at 25 °C (lit.) |
| Solubility in water | slightly soluble |
| log P | 0.72 |
| Vapor pressure | 3.13 kPa (at 25 °C) |
| Acidity (pKa) | 17.5 |
| Basicity (pKb) | 13.6 |
| Magnetic susceptibility (χ) | -5.2E-6 |
| Refractive index (nD) | 1.509 |
| Viscosity | 0.783 cP (25°C) |
| Dipole moment | 1.74 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 56.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | 66.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | −1654 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | D03AX18 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02, GHS07 |
| Signal word | Warning |
| Hazard statements | H226, H301, H311, H331, H373 |
| Precautionary statements | P210, P261, P280, P304+P340, P312, P370+P378, P403+P233 |
| NFPA 704 (fire diamond) | 2-3-0 |
| Flash point | 31 °C (closed cup) |
| Autoignition temperature | 320 °C (608 °F; 593 K) |
| Explosive limits | Lower: 2%, Upper: 41% |
| Lethal dose or concentration | LD50 oral rat 1120 mg/kg |
| LD50 (median dose) | LD50 (median dose) of 1H-Pyrrole: 359 mg/kg (rat, oral) |
| NIOSH | ST0225000 |
| PEL (Permissible) | 200 ppm |
| REL (Recommended) | 200 ppm |
| IDLH (Immediate danger) | 400 ppm |
| Related compounds | |
| Related compounds |
Furan Thiophene Pyridine Indole Imidazole |