Stories about progress in chemical science always capture how much people have shaped new fields through persistent trial and error. 1H-Imidazole-1-Propylamine grew out of tireless exploration in heterocyclic chemistry. Researchers in the mid-20th century understood imidazole rings as important motifs for biological molecules. Their structural curiosity went further, exploring not only the original five-membered ring but also attaching various functional groups like propylamine. This gave rise to compounds with broader utility, including 1H-Imidazole-1-Propylamine, and opened opportunity within both basic research and industry. Early papers reflected a drive to find practical synthetic routes, with improvements in yield coming from tweaks to temperature, catalysts, and purification methods—each lagging behind new ideas until someone found a better way and wrote it up for everyone else to try. The compound now carries a history knitted together by both theoretical interests and hands-on problem-solving in laboratories.
1H-Imidazole-1-Propylamine, sometimes known as N-Propylimidazol-1-amine, stands out for its utility as a building block in pharmaceuticals and specialty chemicals. It combines the nitrogen-rich imidazole ring with a propyl amine tail. This hybrid shape gives it unique binding and reactivity properties. Chemists use it to design drug candidates, corrosion inhibitors, and materials that push performance beyond simple organic amines or imidazoles. Although not always the headline act, compounds like this stay in demand for their versatility during the design phase, and their chemical resilience through downstream processing.
You can usually spot 1H-Imidazole-1-Propylamine by its clear-to-pale yellow liquid form at room temperature. It brings a faint amine-like odor—unpleasant but familiar to seasoned chemists. The molecule measures in at a moderate molecular weight, and its solubility fits what's expected from a compound carrying an imidazole ring and an alkyl chain: completely miscible in polar solvents, less so in nonpolar systems. This profile comes handy during extractions or while running chromatography, letting chemists separate or modify it without major headaches. Given its moderate boiling point, thermal processing asks for some care, but there's nothing exotic about it. The presence of both basic and nucleophilic nitrogen atoms in the same small molecule makes it reactive towards a variety of other reagents in both laboratory and industrial-scale reactions.
In a regulatory context, you’ll often find 1H-Imidazole-1-Propylamine labeled by its IUPAC or common trade names. Chemical suppliers list purity, water content, and sometimes levels of specific byproducts resulting from the synthesis method. Purity levels above 98% often satisfy most commercial and research needs. Material safety data sheets (MSDS) provide a breakdown of hazards, storage advice, and regulatory compliance, echoing the attention to detail that chemical handling demands. Barcodes and QR codes have started showing up on labels in recent years, helping to streamline traceability all along the supply chain.
One tried-and-true route to 1H-Imidazole-1-Propylamine involves N-alkylation. Imidazole serves as the nucleophile, reacting with an alkyl halide such as 1-bromopropane under basic conditions. The resulting N-propylimidazole reacts with ammonia or a suitable amination reagent to introduce the amine function. Some labs prefer a one-pot strategy with high-pressure reactors—this often results in cleaner products and less risk of byproducts sticking around. Purification may combine solvent extractions, distillation, and chromatographic methods, chosen based on the scale and what else is in the mixture. As green chemistry principles gain ground, more researchers look to solvent-free conditions and flow chemistry to reduce waste, especially during scale-up. The reproducibility of these newer routes still relies on thorough routine checks and a healthy dose of practical troubleshooting.
With both an imidazole ring and a primary amine available, 1H-Imidazole-1-Propylamine acts as a participant in a diverse set of chemical transformations. Alkylating agents add more side chains, while acylation or sulfonylation tailors the electronic landscape around the nitrogen atoms. The compound often steps in during the synthesis of imidazole-based ligands used in metal complexation, as well as peptide coupling in organic synthesis. Sometimes, site-specific derivatization of either the amine or the ring nitrogens brings new reactivity patterns. Electrochemical and photochemical approaches have grown in popularity for late-stage modifications, especially for custom molecule libraries in drug discovery. The way this molecule flexes its chemistry makes it a quiet workhorse for R&D teams who crave reliable, well-understood reactions but aren’t afraid to explore fresh ground.
In catalogs and scientific literature, 1H-Imidazole-1-Propylamine appears under names like N-Propylimidazol-1-amine, Propylaminoimidazole, and 1-Propylaminoimidazole. Trade and supply chains in different countries may tag on proprietary or batch-specific codes. This variety can confuse newcomers, but seasoned users cross-check with molecular structure or consult the CAS number for clarity. Over time, manufacturers tend to converge on the simplest, least ambiguous names, though local customs linger in specialty markets.
Responsibility in chemical handling matters as much as technical skill. 1H-Imidazole-1-Propylamine asks for sturdy gloves, eye protection, and good ventilation; exposure can cause irritation or, with bigger spills or splashes, more serious symptoms. Personnel stay alert to the limitations of fume hoods and monitor storage temperatures. Documentation matters—not only for regulatory compliance but to anchor real safety in practice. Chemical compatibility charts and standardized emergency procedures sit within arm’s reach in labs and production spaces, reducing confusion when something does go wrong. Having personally responded to a few minor lab mishaps, I know preparedness cannot substitute for vigilance, but it stops small mistakes spiraling out of control. Training remains the strongest tool for limiting risks—shutdown drills and routine equipment checks build productive habits without fostering complacency.
1H-Imidazole-1-Propylamine stakes its claim across multiple industries. In pharmaceuticals, it serves as a versatile building block for synthesizing bioactive molecules—the imidazole core crops up in antifungals, anticancer agents, and enzyme inhibitors. Chemical manufacturers use it in corrosion-resistant coatings and as an intermediate in dye production. In the crop protection sector, its structure fits as a starting point for exploring new agrochemicals. Industrial water treatment and oil refining sometimes rely on imidazole derivatives for their efficacy in stabilizing systems under demanding conditions. The compound’s signature comes from how it adapts, ready to be molded as research or production goals shift. Its wide footprint reflects that rare blend of chemical resilience and functional diversity, sparking repeat interest from both startups and established players.
From my time working alongside academic groups and smaller industrial labs, the research into 1H-Imidazole-1-Propylamine speaks to the ongoing hunt for molecules that solve difficult problems—from greener synthesis methods to more potent pharmaceuticals. Scientists probe its reactivity, hoping to open up new reaction pathways or unlock properties that competitors lack. Modern R&D tools like high-throughput screening and automation have amplified the pace at which compound libraries grow, letting chemists test dozens or hundreds of imidazole derivatives for biological activity in just weeks. Many of the latest studies explore ways to modify the propylamine tail or swap out ring substituents to tune solubility or target selectivity. Collaboration between synthetic chemists, computational modelers, and medical researchers brings both rigor and surprise results—sometimes an incremental change turns a mediocre scaffold into a breakthrough lead compound. Support from public and private funding reinforces the idea that these building blocks matter, not just for discovery science but for anything downstream that touches health and industry.
Work on toxicity follows strict protocols, sometimes driven by regulatory requirements and sometimes by the ethical backbone of scientific practice. Early toxicological studies focus on the acute effects of skin, eye, and respiratory exposure. Most imidazole derivatives present moderate risks, with irritation or acute toxicity only at higher concentrations. Chronic toxicity, reproductive health, and ecological safety lag behind in published data, so responsible manufacturers invest in ongoing studies to close these gaps. Ecotoxicology draws growing attention, since derivatives can find their way into wastewater streams and aquatic systems. Decision-makers weigh economic benefits against potential harm by looking at both in vivo and in vitro results—benchmarked against thresholds set by health authorities. Some organizations now push for alternative, animal-free models to screen new molecules, and ongoing review keeps protocols current with technology shifts. As new data accumulates, best practices update to reflect both hard evidence and public expectations.
Looking ahead, the prospects for 1H-Imidazole-1-Propylamine look strong—driven by its adaptability across medicinal chemistry, materials science, and greener industrial processes. Continuous improvements in synthetic methods bring higher yields and fewer environmental downsides; adoption of flow chemistry principles reduces waste and streamlines scale-up. High-throughput screening and AI-powered discovery platforms push chemists to design and test analogs faster, bringing new candidates onto the radar that would have gone unnoticed with manual techniques. More attention falls on sustainability benchmarks, including biodegradability and the environmental footprint of supply chains. Market forces reward companies who respond with solutions that cut costs and risks, all while keeping innovation alive. The ongoing interplay between regulation, technical challenge, and the hunt for value-adding functionality shapes a future where 1H-Imidazole-1-Propylamine remains a key player—steady enough to anchor day-to-day work, flexible enough to matter in emergent technology trends.
Step into most pharmaceutical research labs and you’ll find 1H-Imidazole-1-Propylamine somewhere on a shelf. This isn’t one of those molecules that gets all the attention in news stories, but people who work with experimental chemistry know its purpose. Scientists value it as a building block for more complex compounds. This helps streamline the process of creating and testing new drugs. Coronavirus treatments, cancer therapies, and antibiotics — all use similar backbone molecules during research and development. New treatments can’t come together without robust, reliable starting points, and this compound has earned its place in those early stages.
What stands out to me is the way this single substance manages to stretch across disciplines, especially in the hunt for new medicines. I’ve seen lab teams put together libraries of possible new drugs, tweaking functional groups, and this propylamine derivative often pops up. Researchers appreciate its reactivity. The imidazole ring catches their eye because it mimics structures found in living systems. Histamine and histidine, for example, both have imidazole rings. Scientists lean on this similarity when trying to create new molecules that interact with proteins or enzymes related to disease. Try as you might, it’s difficult to swap out every functional group and get the same results, which means this backbone tends to stick around in the world of medicinal chemistry.
In practical terms, chemists reach for 1H-Imidazole-1-Propylamine for more than just drug development. The chemical’s structure allows it to link up with metals, giving it a role to play as a ligand. This makes it a useful partner in catalysis — the behind-the-scenes action of making chemical reactions go faster or more smoothly. Industrial processes that rely on catalysts benefit from this, whether they’re making polymers or working through specialty fine chemicals. The need for efficient chemical transformations continues to fuel interest in molecules like this, which can nudge reactions toward useful products.
For all its benefits, safety always enters the conversation once chemicals leave the lab. Early-stage molecules can pose hazards in manufacturing, transporting, and waste disposal. Researchers and producers grapple with questions around toxicity and workplace safety. Based on my experience in research settings, careful handling, reliable storage, and detailed safety data sheets go a long way. Regulatory guidance helps, but so does a culture of vigilance in chemical management.
Sustainability often lags behind innovation, but not forever. Green chemistry has entered the mainstream in chemical research, and part of my day-to-day work used to revolve around ways to cut down on hazardous byproducts and find substitutes with a lighter environmental footprint. Researchers now hunt for reaction conditions that require fewer resources or less waste, and they continually evaluate whether substances like 1H-Imidazole-1-Propylamine can be made with greener methods or recycled efficiently. Academic and commercial labs work together, trading best practices, nudging the industry toward safer, more responsible use.
Every year, thousands of researchers work behind the scenes with chemicals like 1H-Imidazole-1-Propylamine, but very few people outside those circles ever hear about such compounds. Still, this small molecule helps shape major developments in health, manufacturing, and research. Paying attention to the way fundamental chemicals support innovation, as well as the ways scientists address safety and environmental challenges, sheds light on the often-unseen engine driving progress in biology, health, and industry.
1H-Imidazole-1-propylamine brings together two well-known molecular features—an imidazole ring and a propylamine group. Looking at its basics, the imidazole ring stands out for those in chemistry, especially because you also see this type of ring in histamine and many enzyme cofactors. The ring structure counts five atoms, three carbon and two nitrogen, arranged in a pentagon. In this compound, the propylamine group attaches to one of those nitrogens (specifically the N1 position if you go by standard chemical naming). Each arm of that molecule influences how it behaves and what it can do.
Getting specific, the imidazole ring has double bonds that create electron sharing between the carbons and nitrogens. That gives the ring aromatic stability, sort of like what you find in benzene but with its own chemical twist because of the nitrogens. At the N1 position sits the propylamine chain—a three-carbon stretch with a terminal amine (–NH2). That flexibility makes it easier for the molecule to interact with acidic or basic environments. The backbone looks like this if you break it down in parts: a pentagonal imidazole core, and off its number-one nitrogen floats a –CH2CH2CH2NH2 chain.
The shape and linking of those atoms set the tone for what the molecule accomplishes. I saw in a pharmacology project that imidazole rings often act like tiny magnets, grabbing onto metal ions required for enzyme function or forming strong bonds with biological targets. Adding a propylamine stretches the molecule out, making it better at flowing through basic water-based solutions and interacting with different cell surfaces. That change from a small, flat ring to an extended arm lets chemists tune the compound for tasks in research, medicine, or even materials.
Lots of researchers develop imidazole derivatives hoping to mimic histamine’s role in the body or aiming to block specific enzymes. The propylamine piece brings extra basicity, meaning the molecule can bind opposite charges more strongly or even serve as a starting block for making more complex drugs. For example, this “stretchy” piece can help the molecule cross membranes or hit certain cell receptors more precisely.
Chemists who study medicinal molecules or enzyme inhibitors use the imidazole ring for its stable interactions, which help in building blocks for drugs or enzyme models. By connecting a propylamine, they tweak how sticky the molecule becomes or what chemical partners it attracts. In my own work, a related compound helped control the activity of copper ions in a reaction, all thanks to those nitrogen centers and the flexible propylamine.
This chemical structure also finds a spot in organic synthesis labs. The basic nature means it acts as a platform for new compounds, sometimes anchoring metals or connecting up to bioactive groups. Researchers adjust the propyl group’s length or modify the ring to probe which arrangements give the most useful reactions.
Challenges show up if a compound like this poses toxicity risks or creates by-products in reactions. Chemists keep an eye on purity and side reactions, especially with the basic amine group, since it can latch onto impurities. Safer handling and better analytics reduce risks. That’s where training in chemical techniques, as I’ve experienced in both academic and industrial settings, helps maintain safe, pure outcomes.
To address these concerns, teams invest in improved filtration and purification tools. Careful control of reaction conditions helps limit unwanted effects, and green chemistry protocols aim to minimize waste. Smart design—whether by choosing better solvents, using protective groups, or adjusting synthetic steps—keeps this molecule useful and safe for research and potential drug development.
Many folks who handle chemicals, whether in a lab or at a facility, learn that some rules look strict, but they exist for solid reasons. I remember the feeling of walking into a chemical stockroom for the first time—so many bottles, labels in bold, and a strong scent you can’t quite place. For chemicals like 1H-Imidazole-1-Propylamine, slip-ups in storage rarely end with a minor mess. They chip away at safety, health, and reliability. A friend of mine once dealt with a minor skin burn just because someone left a cap loose. No reason to let it get that far.
1H-Imidazole-1-Propylamine needs a dry, tightly sealed home. Moisture around chemicals like this one jump-starts problems—nobody wants product that degrades or, even worse, forms unwanted byproducts in the bottle. Direct sunlight adds another layer of risk. More than once, I’ve found heat-warped containers left on a sunny bench, contents no longer trustworthy. Opaque bottles or dark storage cabinets help keep the light out, and refrigeration isn’t always needed, but a cool, steady spot on a low shelf away from heat sources does the job.
A neat shelf isn’t about looks. Clear, correct labels stop confusion, which can trigger anything from minor inconvenience to dangerous reactions. Picture yourself searching for the right compound, but the handwriting is smudged or a label missing. That’s no way to work safely. I use tough, chemical-resistant markers and check the inventory each month—no exceptions. Tracking expiration dates catches stability issues before they turn serious. Lost or faded labels mean pulling the container off the shelf—better safe than scrambling after a mix-up.
Mixing imidazoles with strong oxidizers or acids sets the stage for fires or toxic fumes. In my lab days, we never let amine-rich bottles share space with those kinds of substances. One shelf for bases, another for acids, each kept a good distance apart. I remember seeing a minor incident make its way into a research group’s safety bulletin—one wrong shelf assignment, and it took days to air out the room. Strong policy and some basic shelf planning avoid all this.
Working with any amine in a place where air stands still is asking for trouble. Vapors can irritate, spoil the air, or even trigger alarms. I once stepped into a poorly ventilated prep area and left with a sore throat that lasted half the day. Chemical storage rooms work best with a steady flow of fresh air, so invest in a sound ventilation system if you manage a workplace. For small-scale labs, keeping a window cracked or a fume hood running goes a long way.
No one walks into a lab planning a spill, but plans save the day. Absorbent pads line the lower shelves in our room, and everyone keeps gloves in their drawer. Quick access to eyewash stations and showers matters, especially with chemicals that can sting or burn. Letting a spill sit only compounds cleanup and risk. Once, I saw a slow response let a minor leak damage an entire shelf’s worth of stock. Patching the problem fast isn’t just a best practice—it protects budgets and peace of mind.
Every team member should walk through storage protocols more than once. Fresh eyes can spot clutter or confusion. Training isn’t just hand-waving or legal checkboxes; it’s a chance to reinforce smart handling and call attention to new hazards. In my experience, regular training builds pride and encourages people to flag issues before they can cause harm.
Getting storage right isn’t about following orders. It’s about knowing hidden risks and setting up a system that keeps everyone, and the work, on solid ground.
Curiosity about chemical compounds comes up all the time, especially for anyone who’s ever set foot in a lab or worried about what’s in the products they use. 1H-Imidazole-1-propylamine is one of those molecules that sounds complex, but if you look a little closer, it’s just built from atoms we’ve seen a million times before: carbon, hydrogen, and nitrogen. To figure out its molecular weight, you need to know its formula—C6H11N3. Each of these elements has its own atomic mass, and adding them together tells us how much a single molecule weighs in daltons or grams per mole.
This compound has six carbons, which clock in at 12.01 each, for a total of 72.06. Eleven hydrogens, each at 1.01, bring in another 11.11. Three nitrogens, at 14.01 per atom, add 42.03. Put it all together and you land at roughly 125.2 g/mol (grams per mole). For researchers, pharmacists, or chemistry students, nailing this number down lets you work accurately, whether you’re mixing up a buffer or analyzing a new synthesis.
Molecular weight isn’t just a trivia fact. It shapes how substances behave in the real world—from how much water to add to a recipe in the lab, to how molecules will move across cell membranes, to how companies ship and label chemicals. If you get the molecular weight wrong, all sorts of calculations about concentration, dose, or reaction yields take a hit. I’ve seen young researchers scratch their heads over unexpected outcomes in reactions, and more often than not, a sneaky calculation mistake sits at the bottom of the trouble. Knowing the exact figure, like 125.2 g/mol for 1H-imidazole-1-propylamine, gives you a solid base to build off.
Back in my university days, molecular weights could turn into stumbling blocks. Imagine preparing a solution you think is 0.1 molar, only to find the experiment fails because you used the wrong conversion. It’s not pretty, particularly when your professor expects results the next morning. That’s why accuracy matters—not just for grades or reports, but for scaling up reactions in the workplace, producing medicines, or working in any field that leans on chemistry.
In industry, a chemist might calculate how much raw material to order, or what to declare on a safety data sheet. Regulatory agencies in the US, EU, Japan, and beyond expect clear paperwork. Something seemingly basic, like knowing the molecular weight, lands directly in government filing and patent applications.
Digging for the correct numbers demands reliable sources. PubChem, ChemSpider, and the Sigma-Aldrich database lay it out in black and white. Anyone can access these databases—not hidden behind paywalls, not locked in university labs. This gives everyone an entry point, whether you’re a student at a small school or a professional chemist in a high-tech lab.
A single source of truth would cut down on mistakes. Open databases, frequent cross-checks, and clear training help catch errors early. In my experience, double-checking calculations with a colleague or running your numbers past an online tool heads off most trouble. There’s room for schools to keep emphasizing hands-on problem-solving, not just theory. That habit of careful, repeated calculation turns a chemistry student into someone companies trust with their next drug or material. So, knowing the molecular weight of 1H-imidazole-1-propylamine—125.2 g/mol—isn’t just a box to check. It’s part of living up to the standards of reliability, integrity, and accuracy that science depends on.
1H-Imidazole-1-Propylamine often shows up in specialty chemistry labs. It’s a derivative of imidazole, a ring-shaped molecule you’ll run into in many pharmaceuticals, agricultural compounds, and specialty polymers. Some see propylamine chains as building blocks—a sort of Lego piece for scientists hunting new properties or tweaks on existing chemical structures. Anyone who’s spent time in a research lab knows these tweaks can lead to breakthroughs, but they can also bring along safety baggage.
Lots of folks ask: is 1H-Imidazole-1-Propylamine hazardous? Data on this compound remains limited, especially compared to household chemicals. Its close relatives, like plain old imidazole or imidazole derivatives, often irritate the skin, eyes, and respiratory system. If liquid 1H-Imidazole-1-Propylamine contacts the skin, irritation is possible. Breathing vapors or dust during handling sometimes leads to coughing or a scratchy throat, and it’s wise to assume a risk for similar or slightly stronger effects here.
Some chemical suppliers flag this compound as harmful if swallowed. Large-scale data on chronic health effects rarely exist outside of animal testing or cell culture models for chemicals this niche. In those settings, imidazole compounds sometimes show moderate toxicity to lab animals at high doses. Exact numbers for 1H-Imidazole-1-Propylamine toxicity are tough to pin down, so the best rule: treat it with caution and respect.
Work with unfamiliar chemicals always calls for practical safety steps. I remember my grad school days, prepping small vials and pipetting compounds with Latin-sounding labels. More than once, colleagues let their guard down and ended up with rashes or sore throats. For 1H-Imidazole-1-Propylamine, gloves, goggles, and a working fume hood turn into non-negotiables on any bench. Proper storage matters, too—keep containers sealed and chemicals labeled in secure spots.
Accidental exposure most often happens when the basics slip—no gloves, cracked goggles, or spills left too long on the bench. Quick clean-ups and accessible safety showers help limit the fallout. A printed Safety Data Sheet within arm’s reach can be a real lifesaver during emergencies. Ventilation in any chemistry space should handle a little overkill rather than just getting by.
Plenty of hazardous reactions and frustrating accidents spring from small lapses. I’ve seen smart chemists skip recommended gear, assuming a rarely used compound means low danger. Facts say otherwise: niche does not equal harmless. The World Health Organization and American Chemical Society stress preparation and education to prevent avoidable injuries in labs and workplaces.
General chemical literacy empowers people to spot trouble early—familiarity with hazards and thoughtful handling go further than any warning on a datasheet. Open discussions on risk, mandatory safety trainings, and quick access to first aid stations cut incident numbers in half at well-run sites. Students learning basic chemistry sometimes shrug off these warnings until they see or hear about preventable mistakes.
Public health depends on transparency and safety routines, especially as new compounds show up every year. Regulatory agencies call on researchers and manufacturers to share toxicity data and real-world incident reports. Chemists benefit from sharing experiences and learning what works; even a simple “watch out for this one” from a peer can save time and trouble.
Respecting every new chemical as a potential risk doesn’t kill creativity—it keeps people working and learning another day. If you’re handling 1H-Imidazole-1-Propylamine or anything in its family, put safety at the top of your list.
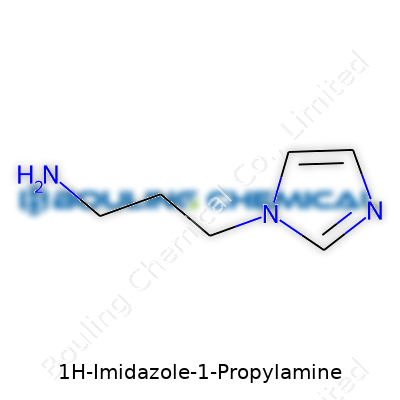
| Names | |
| Preferred IUPAC name | N-(1H-imidazol-1-yl)propan-1-amine |
| Other names |
3-(1H-Imidazol-1-yl)propan-1-amine 1-(3-Aminopropyl)imidazole 3-Imidazol-1-yl-propylamine |
| Pronunciation | /ˈwʌn.ɛm.ɪˈdæz.əˌl.oʊ.wʌn.proʊˈpiːl.əˌmiːn/ |
| Identifiers | |
| CAS Number | 6278-22-2 |
| Beilstein Reference | 136157 |
| ChEBI | CHEBI:16341 |
| ChEMBL | CHEMBL2139973 |
| ChemSpider | 74978 |
| DrugBank | DB08357 |
| ECHA InfoCard | 03b7e4e4-29f0-46f6-8d86-fd1e8224be3a |
| EC Number | 219-264-4 |
| Gmelin Reference | 107520 |
| KEGG | C06384 |
| MeSH | D015719 |
| PubChem CID | 69752 |
| RTECS number | UB9825000 |
| UNII | 2K2D035S1B |
| UN number | UN3241 |
| Properties | |
| Chemical formula | C6H11N3 |
| Molar mass | 112.17 g/mol |
| Appearance | Colorless to light yellow liquid |
| Odor | Amine-like |
| Density | 0.993 g/mL at 25 °C |
| Solubility in water | miscible |
| log P | 0.02 |
| Vapor pressure | 0.232 mmHg (25°C) |
| Acidity (pKa) | 9.36 |
| Basicity (pKb) | 5.90 |
| Magnetic susceptibility (χ) | -59×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.511 |
| Viscosity | 1.06 mPa·s (at 25 °C) |
| Dipole moment | 2.75 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 216.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -20.5 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3747 kJ/mol |
| Pharmacology | |
| ATC code | R06AB05 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS07, GHS05 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H314 |
| Precautionary statements | P261, P280, P305+P351+P338, P405, P501 |
| NFPA 704 (fire diamond) | 2-3-0 |
| Flash point | 72 °C (closed cup) |
| Autoignition temperature | 440 °C |
| Lethal dose or concentration | LD50 oral rat 871 mg/kg |
| LD50 (median dose) | LD50 (median dose): 600 mg/kg (rat, oral) |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 1H-Imidazole-1-Propylamine is not specifically established by OSHA. |
| REL (Recommended) | 0.5 mg/m³ |
| Related compounds | |
| Related compounds |
Imidazole 1-Propylimidazole 2-Propylimidazole 1-Methylimidazole Histamine Imidazole-1-ethanamine |