Long before modern chemical analysis, 1H-1,2,3-triazole appeared as a curiosity among nitrogen-rich heterocycles. Early researchers, navigating the unpredictable and even dangerous world of organic synthesis in the late nineteenth century, often stumbled across triazoles while trying to make completely different things—dyes, explosives, or medicines. It wasn’t until chemists started to map their structures and properties that triazoles gained more attention. With advances in click chemistry over the last two decades, 1H-1,2,3-triazole moved from the background of synthetic methods right into the center of pharmaceutical and materials research. The story behind this compound mirrors the progress of heterocyclic chemistry as a whole: luck favored careful experimentation, academic stubbornness, and a flexible approach to problem solving.
1H-1,2,3-triazole presents itself as a five-membered ring containing three nitrogen atoms in consecutive positions. Its molecular formula is C2H3N3, and the structure packs stability together with chemical reactivity. This combination draws interest from professionals in pharmaceuticals, agriculture, and polymer engineering. Whether prepared at the gram scale for research or in volumes used in bulk synthesis, this molecule shows up under many trade names, both as a pure compound and as part of a larger product. Across industries, triazole substitutions offer a balance between innovation and reliability, due to the molecule’s dependable reactivity and compatibility with other components.
1H-1,2,3-Triazole shows up at room temperature as a white to pale crystalline solid, not quite powdery, and dissolves in water better than most nitrogen-containing rings. Boiling point sits around 203°C, while melting point sits near 120°C. Its smell rarely draws any comment—not much of a scent. In terms of solubility, water, ethanol, and other polar solvents work well, which helps it blend smoothly into reaction mixtures or product formulations. Chemical stability stands out for a five-membered ring with three nitrogens; both acidic and basic conditions might disturb it at extremes, but under most laboratory or industrial procedures, it behaves itself. These properties let chemists handle it in standard glassware with no special precautions beyond those given for energetic or toxic substances.
Pure 1H-1,2,3-triazole arrives with assay values often above 98%, reflecting the ease of purification after synthesis. Commercial samples shame most lab-scale heterocycles in terms of purity. Suppliers label the chemical with its CAS number (288-36-8), its main synonyms, and safety precautions related to handling and storage. A typical container features warnings about skin and eye irritation, directions for storage in a cool, dry space away from oxidizers, and reference to its GHS hazard classification. Companies sometimes add custom packaging to protect workers from dust and spills. Shelf life extends over years if kept under the right conditions—moisture and sunlight do not do triazole any favors.
Old approaches to making 1H-1,2,3-triazole demanded harsh conditions—strong acids, sensitive intermediates, difficult purifications. The explosion in click chemistry, particularly the Huisgen cycloaddition between azides and alkynes, transformed production. Copper(I)-catalyzed approaches now dominate, as they give selectively the 1,4-disubstituted version with high yield and few by-products. Silver and ruthenium have their place in small-scale syntheses or when particular substitution patterns matter, but copper keeps things affordable and scalable. These days, even universities lacking fancy equipment can make triazoles from simple precursors, freeing students or early-career scientists to focus on creative reaction design rather than procedures that merely avoid failure.
The chemistry of 1H-1,2,3-triazole depends on its stability and its slightly acidic NH proton. Basic alkylation installs side chains at the N1 position, letting chemists tailor properties to fit applications ranging from bioisosteres in drug molecules to ligands in transition metal complexes. Electrophilic substitution, though less common, becomes possible in the right hands. Triazole rings also endure a range of conditions that would destroy other heterocycles—strong heat, moderate acids, even many oxidizing agents. This tenacity lets researchers attach them almost anywhere in a larger molecule, knowing that the ring will hold its ground through purification and testing.
As with many long-studied compounds, 1H-1,2,3-triazole travels under a host of names. Textbooks use “triazole” as shorthand, but to avoid confusion with the 1,2,4-triazole isomer, professionals often specify the 1H-1,2,3- designation. Other aliases in catalogs include “1,2,3-triazole” and “azimidaazole,” with the latter cropping up mostly in older literature. Generic pharmaceutical formulations may use “triazolyl” as a functional group. Keep the terminology straight, especially in regulatory documentation or when searching through databases, because the difference of a number or two sometimes separates a fertilizer from a fungicide, or a research tool from an active pharmaceutical ingredient.
You do not want triazole dust flying around, though it presents less danger than many organic chemicals. Skin and eye contact can provoke irritation, inhalation of dust should be avoided, and gloves plus goggles set a smart baseline in laboratories or pilot plants. Material Safety Data Sheets recommend local ventilation and pads or absorbents for accidental spills. In industry, the most serious safety incidents almost always trace back to improper labeling or lax handling of larger volumes—so training and signage matter. No one should treat it casually, nor should storage mix it with strong oxidants or acids. Environmental controls keep waste in check; with triazoles, dilution and controlled disposal routes prevent unintentional contamination.
Pharmaceutical companies rely on 1H-1,2,3-triazole in the making of antifungal agents, blood thinners, and antiviral drugs. The structure pops up in countless molecules, thanks to its resilience to drug-metabolism and its ability to mimic hydrogen-bonding motifs found in nature. In agriculture, its derivatives tackle fungi and bacteria ruining crops, holding off resistance longer than many old-school solutions. Material scientists employ triazoles in polymer backbones, building flexible or conductive plastics, and even in specialty adhesives. In biotechnology, bioorthogonal chemistry leverages triazole rings to label cells or track biomolecules, bridging the gap between chemistry and biology in a way that opens new experiments and techniques.
Research on 1H-1,2,3-triazole focuses on pushing its role in click chemistry ever further. Medicinal chemists keep searching for new designs that exploit the metabolic stability and binding characteristics of triazole rings, rooting out better and safer drugs for cancer, neurological disorders, and infectious diseases. Material scientists chase more sustainable polymers using triazole links, trying for lightweight, fire-resistant, or biodegradable alternatives. Synthetic organic chemistry owes triazole much of its current efficiency, with new catalyst systems emerging yearly, each more selective and cost-effective than before. Academic labs do not hoard these discoveries: journals and patent databases grow thick with open datasets and reproducible protocols, so even small startup teams get a shot at using triazole’s benefits in their products or studies.
Toxicological data for pure 1H-1,2,3-triazole show moderate oral and low dermal toxicity in animal models. Studies highlight concerns about neurotoxicity and impact on the liver at high doses, though such exposures go beyond normal workplace or environmental conditions. Chronic toxicity studies in rodents flag possible risks if triazoles accumulate in the food chain through overuse of derivatives in agriculture. As a result, regulators everywhere demand rigorous environmental fate testing before approving new triazole-based products, and industry groups monitor groundwater and soil levels in regions using these fungicides and pharmaceuticals heavily. Ongoing research by public institutions and company labs aims to clarify risks for both acute and chronic exposure, so safety standards evolve in real time as better evidence and analytical methods appear.
The future of 1H-1,2,3-triazole stretches out past its current domains in chemistry, agriculture, and medicine. Researchers keep asking whether new types of click reactions can simplify drug and diagnostics discovery. Material scientists see opportunities in green chemistry, trying to craft biodegradable or recyclable polymers with triazole links at their heart. Interest in smart drug delivery, nanomedicine, and new energy technologies continue to pull triazole chemistry in unexpected directions. Voices in global health and sustainability look to triazole derivatives for their ability to hold up under harsh conditions without producing persistent pollution. From hands-on lab classes to billion-dollar industry pipelines, this small ring structure offers a rare mix of reliability and inspiration for anyone shaping the next generation of products or therapies.
It’s hard to talk about modern drug design or materials science without mentioning 1H-1,2,3-triazole. I remember the first time I came across this compound: it wasn’t in a specialty chemical stockroom, but in a conversation with a pharmaceutical chemist excited about “click chemistry.” The real appeal here is the triazole ring’s stubborn stability and ease of synthesis. Any lab that needs an efficient way to link different molecules often turns to this reliable five-membered ring.
Pharmaceutical researchers use 1H-1,2,3-triazole as a core skeleton to build new medicines. You see it in antifungals, antivirals, and anti-cancer compounds. This triazole helps drugs resist metabolic breakdown, a real advantage when testing for activity in the body. Take fluconazole for yeast infections or some HIV drugs—many owe their strength and durability to a triazole link. The ring doesn’t just sit there; it makes drugs tougher, helps them stick to enzymes, and sometimes even boosts water solubility.
Anyone who’s been in a synthetic lab has heard “click chemistry.” That phrase is more than a buzzword—it’s a practical tool. The copper-catalyzed reaction between azides and alkynes makes 1H-1,2,3-triazoles with high yields and barely any mess. This approach gave biologists, polymer scientists, and material engineers a way to glue together different parts without a dozen slow, hard-to-clean steps.
I’ve seen chemists tag proteins with fluorescent labels using the triazole as a linking point. Others use it to tie long polymer chains together for new plastics or gels. Each time, the triazole does its job and pretty much ignores water, oxygen, and light, which matters if you want results you can trust.
Beyond labs and hospitals, triazole chemistry makes a difference in farms and textile plants. Farmers rely on triazole-based fungicides to fight crop disease. These pesticides, like tebuconazole or propiconazole, stick around on leaves longer and target nasty mildew or rust in ways that older fungicides cannot. Textile chemists mix triazoles into dyes because the structure handles harsh washing and sunlight—better lasting color on fabric.
It’s not just about making things stronger or longer-lasting. The selective nature of triazole-based drugs and chemicals lets us use less product for the same result. Consider the lower dosages of modern antifungals or the way dye makers reduce waste when their colorants last longer. Reducing side effects—whether for people taking pills or fish swimming downstream from a farm—depends on smart molecule design. Triazoles make it possible.
Every tool has its limits. Overuse of any compound, triazole included, threatens resistance in fungus and weeds or raises new safety questions. Smart regulation, constant monitoring, and research into greener chemistry will keep triazole applications safe. Industry should push for biodegradable alternatives, chemists should share findings with the public, and regulators should update safety guidance as new data comes out. Without those checks, even a superstar like 1H-1,2,3-triazole could wear out its welcome.
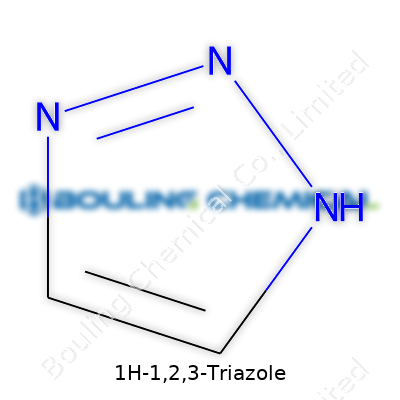
References:1H-1,2,3-Triazole comes down to a simple but striking five-membered ring structure. In the ring, three nitrogen atoms stand together, lining up next to carbon atoms. Think of it like a pentagon: imagine three corners hold nitrogens, and two corners hold carbons. One hydrogen sticks to the first nitrogen, which gives this molecule its unique name. The formula—C2H3N3—doesn’t show off the ring, but chemists picture the nitrogens at the 1, 2, and 3 positions as a kind of backbone for building.
The most common drawing places the hydrogen right on nitrogen number one (hence 1H). The double bonds pop up between the N2 and N3, then between the C4 and C5, making the ring aromatic. Aromaticity doesn’t mean the ring smells; it means electrons flow smoothly through the system, granting some stability and a penchant for taking part in all kinds of reactions.
In my lab days, nearly everyone reached for triazoles at some point. Modern drug discovery would look very different without this compact, nitrogen-rich ring. Medicinal chemists build on the triazole core, tweaking the surrounding groups to hunt for better antibiotics, cancer drugs, and treatments for HIV. The triazole platform creates molecules that hold together under tough conditions and don’t break down quickly, which is vital in medicines meant to last in the body.
Besides pharmaceuticals, 1H-1,2,3-Triazole pops up in agricultural chemicals and corrosion inhibitors, and even as a building block for new materials. One of the practical tricks with this molecule comes from “click chemistry.” This reaction, popularized over the last two decades, locks together molecules to create triazoles efficiently and safely. The reaction often swaps out traditional, harsher chemical methods for something more straightforward and reliable—no special equipment required, just basic copper and common reagents.
Despite all the good, 1H-1,2,3-Triazole is not magic. Sometimes, its preparation brings along by-products or leaves copper residues, which demand careful cleanup. The full story behind the structure also means many related molecules lie in wait; small changes, such as moving a nitrogen or switching a hydrogen, create different isomers. Chemists often sweat the details to make sure they’re picking the right one for the job.
There’s a push now to make these reactions greener. Some research groups swap out metals for bio-friendly alternatives. Others turn to new solvents, or even water, keeping things both safe and efficient. Everybody wins when chemistry sharpens up both its results and its footprint.
Standing back, a small ring like 1H-1,2,3-Triazole proves that structure guides function in both science and the real world. It’s easy to write off a simple molecule, but these rings support new drugs, safer crop treatments, and advanced materials. Progress will depend on squeezing more value from this simple core, reducing waste, and opening doors to healthier and cleaner chemistry everywhere.
I remember walking into my first organic chemistry lab years ago. Rows of reagents with names meant nothing at first glance, but every single label hid critical safety concerns. Fast forward to today, and 1H-1,2,3-triazole gets tossed around a lot in research and specialty synthesis. It plays a big role in click chemistry, which made a splash in drug discovery. Its versatility tempts people to treat it with less caution than bigger, tougher-sounding names. But safe handling doesn’t only belong to the “really dangerous” substances.
Not many folks outside the lab world know about 1H-1,2,3-triazole. On paper, it’s a small, nitrogen-rich ring; in practice, it packs quirks you can’t ignore. According to the GHS (Globally Harmonized System), this compound often comes with warnings about irritation and possible allergic reactions if you happen to get it on your skin or in your eyes. The American Chemical Society’s working group points out triazoles as respiratory irritants. You spill some, and your hands don’t feel right. That burning isn’t just imagination.
From my own use, gloves—nitrile, not latex—prove non-negotiable. Chemical-resistant goggles matter as much as your lab notebook. I once skipped them for "just a quick weigh-out" and paid with watery eyes all morning. It only takes one mishap to respect the rules.
Ventilation stays at the core of chemical safety. Any time I open a container of triazole, I stick close to a fume hood. Many university safety data sheets call for it, and I’ve found the hood saves both your lungs and your peace of mind. Even with low volatility, dust or small particles get airborne, fast.
Storage demands dry, sealed containers—moisture encourages unwanted reactions, and 1H-1,2,3-triazole shouldn’t sit near oxidizers or acids. Years ago, a colleague in a neighboring lab ignored that advice, and a mislabeled shelf led to cross-contamination. Luckily, our safety officer spotted the error before anything caught. Lessons like that reinforce why proper labeling goes beyond bureaucracy.
Disposal doesn’t mean tossing it down the drain. Local regulations vary, but hazardous waste protocols need following. Most research institutions carry approved disposal channels; home chemists face stricter limits. Even minor quantities demand respect, as environmental buildup threatens aquatic life. I read a 2022 study showing traces of related triazoles in river samples—no one wants their hobby or research polluting the water table.
Guidelines only go so far. The real trick turns up in habits. Peer checks, written protocols, and ongoing training keep safety standards from turning into background noise. The strongest labs treat every bottle as a risk—an approach that pays off, especially in settings with rotating students or lower budgets. Supervisors who walk the talk, model glove usage, and reward good habits change attitudes for the better.
Whether someone works at a university, in industry or for themselves, dealing with 1H-1,2,3-triazole means balancing its benefits with common-sense care. In my experience, no shortcut or assumed exception justifies skipping precautions. A little hassle now blocks a lot of pain—or paperwork—later.
Medicinal chemistry teams see fresh directions with 1H-1,2,3-triazole. One reason: it slips into drug designs easily, replacing parts that are unstable or prone to quick breakdown in the body. Many antifungal and antibacterial drugs rely on this trick. Ritonavir, an HIV medicine, sports a triazole ring. These rings also help build cancer drugs and experimental antivirals. Chemists count on triazoles to bring reliable hydrogen bonding and improved metabolic stability into a new compound. Lab notebooks around the world are full of these tweaks, trying triazoles in spots where past molecules fell short.
"Click chemistry" comes up again and again for good reason. The copper-catalyzed azide-alkyne cycloaddition, discovered by Sharpless and Meldal, uses triazole formation as its main feature. Scientists love this because it runs fast, doesn’t require harsh conditions, and works in water. I’ve seen it take a complicated synthesis and make it feel like snapping together LEGO pieces. This trick opened the door for bioconjugation: linking fluorescent dyes to DNA strands, putting drugs on antibodies, and creating tailor-made polymers. Research groups making new biomaterials lean on this reaction, opening a playground for advances in diagnostics and targeted therapy.
Anyone who follows agriculture knows triazole-based pesticides keep showing up on product labels. Triazole fungicides handle stubborn plant diseases. Farmers fighting powdery mildew or rust often reach for compounds like tebuconazole or propiconazole. These crop protection agents keep yields steady when unpredictable weather threatens to bring blight. Their use supports food security, a real concern as climate and global populations shift. While discussions about site contamination and resistance are ongoing, the role of triazoles in protecting harvests continues to matter for global supply chains.
Polymers built on triazole links resist heat and chemical attack, turning up in specialty coatings, adhesives, and engineering plastics. I’ve seen materials engineers turn to triazole chemistry once the usual polyurethanes and epoxies hit a wall. These polymers end up protecting sensitive electronics, lining chemical reactors, and even making strong dental materials. The same “click” reaction from earlier now helps link long chains or stick new groups onto surfaces. It's a handy way to improve material performance without dangerous solvents or hard-to-control reactions.
Triazole groups are sneaky helpers in chemical sensors. Whether for detecting metal ions or checking for environmental toxins, the triazole's nitrogen atoms latch tightly onto targets. Labs use this feature in chemosensors and as tags for imaging. Some blood tests use triazole-based probes to check biomarkers, improving health monitoring from hospitals to remote field clinics. The selectivity of these sensors traces back to the unique “grip” the triazole ring holds over its target molecules.
It pays to look at both progress and caution with triazoles. Toxicity, persistence, and risk of resistance in agricultural and medical arenas need better tracking. Some nations have tightened rules on certain triazole chemicals, spurring better design for safer, faster-degrading analogues. In the lab, green chemistry adaptations aim to replace copper or lower byproduct loads. These efforts need broad support from funding agencies, regulators, and private industry if the balance between usefulness and risk will stick.
Every now and then, in the world of chemical research and industrial work, a name like 1H-1,2,3-Triazole pops up. It’s a regular in the toolbox of chemists thanks to click chemistry and drug discovery. Yet, people rarely talk straight about how to keep these chemicals safe and stable. Long story short: 1H-1,2,3-Triazole needs a cool, dry, and well-sealed home. Skip one of these, and you might end up with a degraded sample or a safety concern.
I’ve spent enough time pouring over reagent bottles and inventory sheets to know this: moisture and heat will ruin a good stock of triazole faster than anyone likes. Triazoles don’t combust spontaneously or react violently under normal conditions. Still, long exposure to warmth and open air brings a slow breakdown. Fungi and bacteria love warm, moist spaces. Storing this compound tightly closed inside a dry, amber glass container, in a refrigerator or climate-controlled chemical cabinet, keeps it out of trouble. Light and air let unwanted chemical reactions sneak in.
Standard lab glass bottles with airtight seals work perfectly. Avoid polyethylene or other plastic containers for longer-term holding; triazoles sometimes absorb trace contaminants from plastic. I learned that lesson in a routine experiment checking purity—plasticizer peaks showed up in the chromatogram. Always prefer amber or opaque containers: some synthetic triazoles display sensitivity to light, which speeds up decomposition.
Shelving height isn’t just for show. Keep the container on a lower or middle shelf, away from the edge. Accidents happen. A bottle falling off the top shelf brings broken glass, triazole powder everywhere, and a nasty clean-up job.
Triazole’s shelf life often reaches up to two years in factory-sealed form, stored cold and away from sunlight. Once opened, plan to use up the contents within a year, if not sooner. Chemical suppliers often stamp a “best before” date, but don’t just trust the label. Sometimes, a check by NMR or HPLC tells a truer story about purity.
Humidity inside the room and how often you break the seal on the bottle both chip away at shelf life. Repeated opening invites oxygen and moisture. One study out of Japan tracked decomposition of triazoles over 18 months—well-sealed samples in cool, dark places kept over 95% purity, but those left at room temp or exposed to air dropped below 80%. For people who make triazole regularly, it’s eye-opening to see the difference a closet versus a fridge can make.
Every good lab log should include chemical receipt, opening date, and test results after long storage. This way, nobody touches an old bottle without checking its record first. If something looks clumped or off-color, toss it with the proper procedure. Never mix new stock with old—cross-contamination ruins the point of proper storage.
Smart practices help catch problems before batches go bad. In my experience, staying consistent with storage pays off: pure triazole convinces everyone, from the bench chemist to the regulatory inspector, that quality comes from care—not luck.

| Names | |
| Preferred IUPAC name | 1H-1,2,3-triazole |
| Other names |
Trazole Azimazole 1,2,3-Triazole 1H-1,2,3-Triazole |
| Pronunciation | /ˈwʌn eɪtʃ ˈwʌn tuː θriː traɪˈæzoʊl/ |
| Identifiers | |
| CAS Number | 288-36-8 |
| Beilstein Reference | 120924 |
| ChEBI | CHEBI:35585 |
| ChEMBL | CHEMBL1165 |
| ChemSpider | 86111 |
| DrugBank | DB02142 |
| ECHA InfoCard | 03e2fe2a-3ac0-4adc-870b-18807b614269 |
| EC Number | 202-300-9 |
| Gmelin Reference | 79068 |
| KEGG | C06591 |
| MeSH | D014286 |
| PubChem CID | 13524 |
| RTECS number | XAG3675000 |
| UNII | 24S4N2UQ1I |
| UN number | UN1992 |
| Properties | |
| Chemical formula | C2H3N3 |
| Molar mass | 69.07 g/mol |
| Appearance | White to off-white crystalline powder |
| Odor | ammonia-like |
| Density | 1.19 g/mL at 25 °C(lit.) |
| Solubility in water | Miscible |
| log P | -0.47 |
| Vapor pressure | 3.8 mmHg (25°C) |
| Acidity (pKa) | 9.3 |
| Basicity (pKb) | pKb = 8.9 |
| Magnetic susceptibility (χ) | -40.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.503 |
| Viscosity | 0.837 cP (20°C) |
| Dipole moment | 1.84 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 106.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | 140.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -860 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | D01AE15 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Danger |
| Hazard statements | H301 + H311 + H331: Toxic if swallowed, in contact with skin or if inhaled. |
| Precautionary statements | P261, P280, P305+P351+P338, P304+P340, P312 |
| NFPA 704 (fire diamond) | 1-3-0 |
| Flash point | 23 °C (closed cup) |
| Autoignition temperature | 220°C |
| Explosive limits | Explosive limits: 7-45% |
| Lethal dose or concentration | LD50 oral rat 1,280 mg/kg |
| LD50 (median dose) | LD50 (median dose): 1,280 mg/kg (rat, oral) |
| NIOSH | BZ2060000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | REL (Recommended Exposure Limit) of 1H-1,2,3-Triazole is: "10 mg/m3 |
| IDLH (Immediate danger) | IDLH: 500 mg/m3 |
| Related compounds | |
| Related compounds |
1,2,4-Triazole Pyrazole Imidazole Tetrazole Benzotriazole |