In the early 20th century, chemists started looking beyond basic solvents, searching for functional monomers with strong solubility and polymerization potential. 1-Vinyl-2-pyrrolidone first came to the spotlight when German researchers, keen on pushing polymer science forward, synthesized it around the time nylon was drawing global attention. Since then, the compound quietly moved from obscure lab benches to bulk manufacturing settings due to its versatility. Industrial use grew after World War II, when demand spiked for polymers with better solubility in water, leading to broader interest in polyvinylpyrrolidone (PVP), which starts with this monomer.
1-Vinyl-2-pyrrolidone, sometimes called N-vinylpyrrolidone, stands out thanks to its ability to form high-molecular-weight polymers. Unlike some monomers that bring complications or demand tricky handling, it blends well with a wide range of solvents and reactants. Producers sell both technical and higher-purity grades, depending on whether it's destined for cosmetics, pharmaceuticals, or industrial adhesives.
This monomer appears as a clear or pale yellow liquid, with a faint odor that can grip the nose if you work with open containers. It blends quickly into water, alcohols, and most polar organic solvents, which makes cleanup and formulation straightforward in labs. With a boiling point around 90°C at reduced pressure and a melting point under typical room conditions, it stays liquid year-round in ambient storage, which helps avoid production bottlenecks from solidification. One defining property is its reactive double bond on the vinyl group, hanging readily off the pyrrolidone ring, making it eager for radical polymerization but also introducing a need for measured storage to prevent runaway reactions from light or heat.
Manufacturers focus on purity, water content, color index, and residual inhibitor levels. Most batches for regulated industries report purity above 99%, water usually below 0.2%, and color numbers less than 50 Hazen. Clear lot tracking goes hand-in-hand with shelf-life data, as improper storage lets the monomer degrade or polymerize prematurely. Safety data sheets post hazard codes for skin and eye irritation, along with advice for spill cleanup to protect both users and the environment. Labels often include chemical names, batch numbers, storage advice, and warnings to keep from light and away from incompatible materials like strong oxidizers.
Preparation generally starts from γ-butyrolactone, running through amination to produce 2-pyrrolidone. The final transformation uses strong base and acetylene to bring the vinyl group onto the nitrogen. Since high-purity product demands careful operation, process engineers design reactors and purifiers that minimize exposure to air or impurities, which could contaminate a whole lot. Manufacturers invest in in-line controls and regular sampling to keep to spec, because a slip in catalyst or reactant cleanliness can ripple downstream, causing stability or color shifts.
The vinyl group’s reactivity supports a host of additions and copolymerizations. Free-radical polymerization dominates industrial use, especially when making PVP, but chemists have pushed modifications with quaternization, ring opening, or cross-linking too. Copolymerization with acrylic acid, vinyl acetate, or other hydrophilic monomers means manufacturers create resins tailored for hairsprays, medical adhesives, or water treatment. Even the pyrrolidone ring itself can react under acidic or basic conditions, opening up paths to derivatives suited for specific end-uses.
Besides the systematic IUPAC label, suppliers and journals call it N-vinylpyrrolidone, sometimes shortened to NVP. In catalogs, “vinylpyrrolidone” also shows up. Polyvinylpyrrolidone (PVP)—the main polymer—draws most recognition, but all these names point back to the same monomer. Common trade names include Luviskol, Polypore, and Plasdone, depending on the manufacturer and product form.
Long experience reminds us not to cut corners with safety on reactive monomers. Contact with skin tends to irritate, and splashes can sting the eyes. Proper PPE, ventilated storerooms, and drum-handling procedures protect workers from both exposures and spills. Fire codes classify 1-vinyl-2-pyrrolidone as flammable, so facilities store it in explosion-proof buildings, well away from ignition sources. Those handling the chemical adapt policies in line with region-specific regulations like OSHA in the U.S. or REACH in Europe, making audits and traceability real priorities. Discharge to wastewater draws environmental scrutiny, so most plants treat or incinerate residues instead of direct dumping.
Manufacturers use 1-vinyl-2-pyrrolidone for more than polymer production. Its water solubility means it works as a dispersant in inkjet inks, giving smooth lines and bright colors. PVP, derived from this monomer, thickens pharmaceuticals, binds tablets, stabilizes beer foam, clarifies wine, and even holds hair in place. Adhesives made from copolymers stick to skin without causing allergic reactions, making them ideal for bandages and transdermal patches. When added to hydrogels for contact lenses, the flexibility and comfort improve over older materials. Water-treatment additives built with N-vinylpyrrolidone derivatives capture metals and slow down scale buildup, helping industry and municipalities both.
Lab groups and startup companies invest time in understanding not just the polymerization chemistry, but also green process improvements. One area getting attention focuses on recycling or efficient conversion byproducts to minimize waste. Researchers dig into controlled or “living” radical polymerization methods, hoping to tailor polymer architecture and performance, making next-generation adhesives and medical products. Digital modeling aids in predicting how NVP-derived copolymers behave under stress, cold, and UV exposure. Published studies highlight drug delivery, with some teams figuring out how to load sensitive therapeutics into PVP matrices that shield actives and deliver them predictably in the body.
Animal testing, now under close ethical oversight, and in vitro assays both point to a need for caution but don’t raise red flags at typical exposure levels. The monomer itself causes irritation with direct skin or eye contact, and workers exposed to high concentrations over long periods may experience headaches, or discomfort. Studies in rodents using high doses showed some organ effects, but the no-effect levels rest far above what most workers or consumers face. Modern safety data from regulatory agencies, including the U.S. EPA and European Chemicals Agency, show that, handled under proper restrictions, the risks stay manageable. Finished PVP—the backbone of tablets and cosmetics—shows very low toxicity, though monomer residues in end products need monitoring to avoid long-term accumulation.
Polymer chemistry pushes forward because industries demand more from materials—medical device makers ask for stronger, less reactive coatings; electronics need binders able to handle new pigments; agriculture benefits from formulations that release nutrition or pesticide slowly. Each of these fronts finds something to gain from advances in N-vinylpyrrolidone technology. With the rise of biodegradable plastics, specialists look at how to tweak the molecule or polymer chain so water, heat, or light break them down safely after use. Researchers push toward catalytic processes that work under milder conditions, lowering energy use and minimizing toxic byproducts. The focus keeps industries on track to meet regulatory and sustainability targets, without giving up performance. The next round of improvements—alternative raw sources, superior purification, smarter copolymer design—will rely on the same deep understanding of this unassuming, hard-working monomer.
Some chemicals lead fairly quiet lives, sitting on a shelf or working behind the scenes. 1-Vinyl-2-pyrrolidone, or NVP, is one of those. The name might trip up most of us, but the jobs it does make a difference almost every day.
You probably won’t see NVP promoted on product packaging, but it’s built into plenty of items. NVP serves as a monomer—basically, it’s a building block chemists snap together to form polymers. The most common one made from NVP is polyvinylpyrrolidone (PVP). This polymer finds its way into many products because it dissolves in water and bonds well with a range of other materials.
PVP takes on a starring role in pharmaceutical tablets. Drug makers appreciate how it binds active ingredients together, helping pills keep their shape and hold together through transport. Unlike a lot of chemical binders, it breaks apart quickly in stomach fluid, giving fast drug release. PVP has earned its spot as a pharmaceutical workhorse, and none of that happens without NVP at the start.
Think back to a bottle of hair spray you’ve seen—or actually used. Many sprays rely on film-forming agents that make hair stay in place long after you walk out the door. That film often comes from PVP, made possible by NVP. Toothpaste, shampoo, and skin creams also bring in PVP for its ability to mix oily and watery parts together. The result? Products that don’t separate in the tube, which means less shaking and much smoother use.
Printing technology has changed over the years. NVP again helps out, this time in inks and coatings. It gives colorants a stable, even dispersal over surfaces, so the final print looks sharp and resists fading. Anyone who’s struggled with streaky ink knows how important that can be.
Coatings and adhesives use PVP for similar reasons. In electronics, for example, circuit board makers rely on this property for thin, even films that survive intense fabrication steps. PVP also works as a glue in specialty papers and packaging.
Imagine a hospital trying to get clean, reliable test results without contamination. PVP-based medical formulations, including solutions for disinfecting and wound care, help provide that level of sterility. Some surgical gloves and medical devices benefit from coatings built on PVP, providing a smooth, low-allergy surface that doctors can trust to perform.
1-Vinyl-2-pyrrolidone doesn’t come without questions about waste, toxicity, or environmental release, especially because large-scale industrial production keeps rising. Manufacturers face the task of reducing byproducts and preventing chemical leaks into water and soil. The best-run plants rely on closed-loop systems and rigorous safety monitoring. Investment in research for greener synthesis matters. Customers should support companies that publish their safety data and strive for cleaner processes, not just low prices.
Public health and environmental watchdogs continue to push for stricter controls on substances like NVP at the production level. Workplace exposure limits, end-to-end tracking, and transparent labeling play strong roles in keeping staff and communities safe. Major manufacturers that take these measures seriously usually set better examples, building trust and long-term value.
NVP represents an often-overlooked foundation for everyday products. From medicine to printing, to personal care, users count on its reliability. But the push for safety and sustainability should remain a priority if its benefits are to last.
Most folks outside of research and chemical manufacturing probably never hear about 1-vinyl-2-pyrrolidone, but this chemical turns up in more places than many realize. Used in making polymers for cosmetics, adhesives, and even some inks, its reach stretches farther than lab benches. That being said, it’s easy to overlook the questions around safety, especially given how common it shows up in day-to-day products.
Sometimes it seems easy to trust the stuff that ends up in household goods, but research on 1-vinyl-2-pyrrolidone paints a story that’s more complicated. According to the European Chemicals Agency, this chemical causes serious eye irritation and skin irritation when not handled right. They classify it as a substance that can cause allergic skin reactions, a concern for manufacturers and anyone working directly with concentrated forms.
The U.S. Environmental Protection Agency studied potential long-term impacts, flagging possible DNA damage in animal studies—a sign that repeated or high exposure brings risks regular consumers might not realize. There’s no strong evidence of cancer in people just from using products that contain trace amounts, but the World Health Organization keeps encouraging scientists to track long-term effects, especially among workers.
In the workplace, problems often start with breathing in vapors or getting repeated skin contact. Protective equipment, good ventilation, and safe handling protocols all help reduce those risks. At home, the exposure stays much lower; the chemical is usually baked into plastics, resins, or other stable materials. Still, allergic reactions don’t require high doses, and people with sensitive skin have reported issues after using cosmetics with high concentrations.
From my own experience talking with lab technicians in the industrial coatings sector, personal stories highlight another concern: the gap in training. People new to the job sometimes don’t fully grasp why gloves and goggles are non-negotiable. One technician shared how a brief splash led to a painful rash and missed workdays, even though the safety sheets spelled out the danger.
Government regulators don’t pull chemical approvals lightly. Safety studies, workplace monitoring, and rules around permissible limits keep public exposure in check. For example, OSHA and the EU’s REACH program both lay out strict labeling and handling requirements. But it relies on companies to uphold those standards in practice.
Consumer watchdogs continue pushing for more transparency. Ingredient lists often use chemical trade names that mean little to the average shopper. Expanding clear labeling, especially in cosmetics, gives individuals better ways to avoid ingredients that trigger reactions.
For average folks, checking cosmetics or personal care labels helps avoid skin reactions. People working with the chemical need solid training, protective gear, and well-maintained ventilation. Companies carry a responsibility that goes beyond legal minimums: proper storage, up-to-date safety information, and health monitoring can make a difference.
The story of 1-vinyl-2-pyrrolidone shows the link between invisible risks and everyday safety. Real protection doesn’t come from fear, but from the details—reading, asking questions, and keeping workplaces honest about what’s really in the air and on our skin.
1-Vinyl-2-pyrrolidone finds its way into all sorts of industrial settings because it boosts performance in adhesives, coatings, and personal care products. I remember seeing a drum of this stuff tucked away in a warehouse, looking almost harmless, but experience taught me quick: chemical safety never takes a backseat. This monomer isn’t just another jug on a shelf—it reacts to heat, air, and light with surprising speed. Anyone storing chemicals owes it to themselves and their crew to grasp exactly what that means.
It’s tempting to treat storage like an afterthought, especially when the daily grind puts pressure on space and time. The trouble with 1-vinyl-2-pyrrolidone? It polymerizes if it sits somewhere too warm or if oxygen sneaks in past a weak seal. If you’ve ever walked by drums that felt a little warm to the touch, you know how a slight spike in storage temperature can spell trouble—polymers clogging valves, ruined stock, safety risks all around. Data agrees: industry reports point to temperature control and air exclusion as key to keeping this chemical stable. Even a well-intentioned shortcut like using clear containers or skipping a lid can cause the entire batch to degrade fast.
A cool, dry, shaded place forms the baseline. I’ve seen best results keeping drums at 15–25 degrees Celsius, away from sunlight and away from anything that generates heat—like steam pipes, heaters, or power tools running nearby. It doesn’t just help the chemical last; it cuts down spoilage and costly disposal. Anyone who ever handled a drum that polymerized too soon knows there’s nothing easy about cleanup or getting rid of the mess safely.
Choosing the right container matters, too. Stainless steel or high-density polyethylene tanks with tight-fitting lids work well. Oxygen is the enemy here, so a nitrogen blanket or simply minimizing headspace cuts down on the chances of unwanted reactions. I’ve watched teams use cheap seals or scuffed-up drums to save money in the short term, but those moves usually cost more. Every chemical handler can tell a story about a budget container leaking—and the headache that follows.
Labeling remains one of the simplest ways to avoid disaster. A clear tag with the substance’s name, hazard warnings, and the date received stops mix-ups—no one wants to grab the wrong drum and end up with a runaway reaction. It helps to keep emergency spill kits and eye-wash stations close, since getting this chemical on skin or in eyes can sting badly.
Some folks think that just following basic guidelines is enough, but I’ve found regular checks and maintenance make the real difference. Leak checks, container inspections, and reviewing expiry dates become second nature in busy warehouses, and it pays off every time. I’ve worked in places where one overlooked drum became a major cleanup operation.
Switching to smaller containers and using inventory management systems has kept stocks fresher. Nothing beats technology that alerts you to fluctuations in storage conditions. I’ve seen more sites investing in automated monitors so temperature and oxygen levels never slip out of range.
Bringing in regular staff training closes the loop. Training leads to smart habits: sealing every drum, checking dates, and reporting odd smells or bulging lids. Over time, this culture of care keeps everyone safer and saves money—no one wants to scrap a batch worth thousands because someone forgot to screw down a cap tightly.
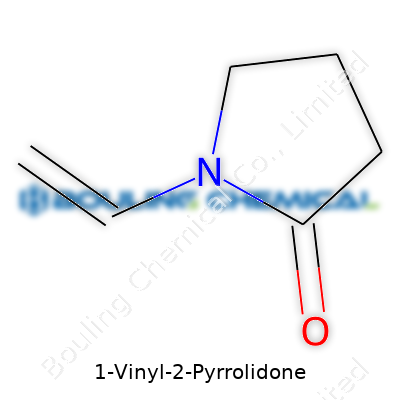
1-Vinyl-2-pyrrolidone, often called N-vinylpyrrolidone by folks in the chemical industry, carries the formula C6H9NO. The structure is straightforward yet clever: there's a five-membered ring with four carbons and one nitrogen, known as a pyrrolidone ring, with a vinyl group sticking off the nitrogen atom. This vinyl group can look like a side branch: a simple double-bonded carbon chain that makes the molecule ready to take part in polymerization. With this small twist in the structure, you get a compound that brings notable versatility to the production floor.
So, the layout goes like this: the backbone is the pyrrolidone ring, a lactam ring formed when gamma-aminobutyric acid cyclizes. A vinyl group (-CH=CH2) latches onto the nitrogen, replacing what would otherwise be a hydrogen. Chemists sketch it with the five-atom ring on one end and a two-carbon chain placing the double bond out front, making the whole molecule both stable and surprisingly reactive.
Anyone who’s worked with monomers understands the vinyl group’s importance. With that reactive side chain, the molecule makes an ideal candidate for polymer chemistry. This isn’t just trivia. Polyvinylpyrrolidone (PVP), a polymer that manufacturers whip up from this compound, is behind countless everyday products—from hair sprays to tablets. Working in a formulation lab brought home to me just how much a single tweak in the molecular structure can mean for performance. A vinyl hook transforms a benign ring into the foundation for something bigger. If you’re involved in doing hands-on chemistry, you learn quickly that these seemingly small changes drive the larger utility of the material.
The presence of the lactam ring and the vinyl group means the molecule isn’t just easy to polymerize; it also brings some safety needs. Inhaling or ingesting pure monomer calls for caution—exposure above certain levels isn’t safe, especially in workplaces handling this chemical by the drum. Exposure control and best practices have to take priority, not only for personal safety but to ensure a high quality, impurity-free product that meets industry standards.
On the ground, labs see challenges with purity of the monomer. Impurities like water or peroxides can set off unwanted reactions. Reducing these risks takes well-thought-out process controls. In facilities I’ve worked in, vacuum drying and nitrogen blanketing go hand-in-hand with regular monitoring. Downstream, the applications for 1-vinyl-2-pyrrolidone stretch from medical devices to inks. Each use demands a close look at the structure-function relationship, as impurities or off-ratio compositions quickly affect final quality and safety.
By focusing on small changes—like the addition of a vinyl group—chemists open up a world of new possibilities. Experience working with both the raw monomer and the resulting polymer shows how tightly structure and performance connect. Getting the basics right means giving future innovations solid ground to stand on—whether for sustainable manufacturing, advanced pharmaceuticals, or new materials that handle water or heat better than before.
1-Vinyl-2-Pyrrolidone pops up in labs and factories, especially anywhere you see the making of polymers or specialty inks. This clear liquid can sneak into the air by just hanging around in an open container or spill. If you've ever walked through a chemical storage room and caught that distinct odor, compounds like this often cause it. My own work with lab chemicals taught me to respect substances you barely notice—often, those are the ones that matter most for safety.
Most folks who work with chemicals know a careless moment at work means taking chemicals home in unexpected ways—on a shirt cuff, skin, or even shoes. While the Material Safety Data Sheet covers the core points—irritation risk, potential effects on organs if absorbed over a long time, and fire risk—these bullet points sometimes feel abstract until you see a co-worker struggle with a chemical burn or develop allergies from repeated skin contact.
Gloves aren’t just for show. The right glove—a nitrile or neoprene model, not just latex—keeps solvent penetration to a minimum. Eye shields with splash guards do more than just fog up your vision; they block those surprise splashes that show up when you least expect it. I remember my mentor in chemical engineering saying he only forgot goggles once, and he never risked it again after rinsing his eyes for what felt like hours.
Ventilation should not be an afterthought. Even small open bottles will release vapors. Fume hoods, properly working exhaust, or at least local extraction go a long way to protect lungs and keep the smell from clinging to clothing. I’ve visited workplaces where open containers sat by the window, relying on a draft. That’s not enough. One should always have the air moving in the right direction and filtered, not just out the window.
For accidental spills, absorbent pads or a dedicated spill kit beat paper towels and tap water any day. Keeping chemicals in sealed, clearly labeled containers isn’t just a habit for neat freaks; it keeps bottles from cracking or leaking, especially in changing temperatures. Ensuring containers have a secure cap and get stored in cool, dry spots helps avoid pressure build-ups and accidental openings.
Training can’t stop at the quick slideshow or a dusty safety manual checked off on day one. Hands-on refreshers matter more than paperwork. Employees should expect regular drills and hands-on demonstrations. Companies respecting their workers invest in training, not just compliance. This attitude extends to investing in real-time detection, improved PPE, and easy access to eyewash stations or emergency showers.
Taking chemical safety seriously means asking tough questions: Are there safer alternatives to this substance for the current application? Does the protective gear really fit, or is it just the cheapest option? Are there gaps in how people answer spills or in how often safety equipment gets checked?
In the day-to-day, true safety relies on routine. You don’t just check your gloves at the start of a new bottle—you check every time. Keep emergency numbers visible, spill kits stocked, and make sure a new lab tech learns hands-on how to don protective gear. Only real habits and preparedness—the kind you don’t have to think about—create a work environment where everyone heads home safe, no matter what chemicals the day brings.
| Names | |
| Preferred IUPAC name | 1-ethenylpyrrolidin-2-one |
| Pronunciation | /waɪˈnɪl tuː paɪˈrɒlɪˌdoʊn/ |
| Identifiers | |
| CAS Number | 88-12-0 |
| Beilstein Reference | 120924 |
| ChEBI | CHEBI:51813 |
| ChEMBL | CHEMBL14037 |
| ChemSpider | 8218 |
| DrugBank | DB13925 |
| ECHA InfoCard | 03e393b7-c193-4339-a3e9-6fa7a13606d4 |
| EC Number | 212-134-7 |
| Gmelin Reference | 10459 |
| KEGG | C06590 |
| MeSH | D014807 |
| PubChem CID | 7977 |
| RTECS number | UY7175000 |
| UNII | 609F0KUA1S |
| UN number | UN2308 |
| Properties | |
| Chemical formula | C6H9NO |
| Molar mass | 111.14 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | slight amine-like odor |
| Density | 1.03 g/cm3 |
| Solubility in water | miscible |
| log P | -0.37 |
| Vapor pressure | 0.38 mmHg (25 °C) |
| Acidity (pKa) | 16.90 (at 25 °C) |
| Basicity (pKb) | -1.2 |
| Magnetic susceptibility (χ) | -54.3·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.512 |
| Viscosity | 2.04 mPa·s (25 °C) |
| Dipole moment | 4.09 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 322.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -117.4 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2568.7 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07, GHS08 |
| Pictograms | GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P210, P264, P273, P280, P305+P351+P338, P337+P313, P370+P378 |
| NFPA 704 (fire diamond) | 2-1-1 |
| Flash point | 91 °C |
| Autoignition temperature | 425 °C |
| Explosive limits | 1.4-11.1% |
| Lethal dose or concentration | LD50 oral rat 3730 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat 6,720 mg/kg |
| NIOSH | WN 293 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 1-Vinyl-2-Pyrrolidone: Not established |
| REL (Recommended) | 1 ppm |
| IDLH (Immediate danger) | 250 ppm |
| Related compounds | |
| Related compounds |
2-Pyrrolidone N-Vinylpyrrolidone polymers (PVP) N-Methyl-2-pyrrolidone Pyrrolidine |