After World War II, chemical research exploded with new ideas about molecular frameworks and functional group manipulation. The arrival of fluorinated compounds like 1-Tert-Butyloxycarbonyl-4,4-difluoropiperidine shared in that momentum. Early work explored how inserting fluorine atoms into key positions on the piperidine ring would shape both stability and reactivity. Many chemists, myself included, have watched the interplay between protection group strategy—like tert-butyloxycarbonyl (Boc)—and fluorine substitution pull new possibilities out of organic reactions that otherwise stayed inert. This intersection of protection chemistry and ring modification didn’t grab headlines, but it quietly gave medicinal and synthetic chemists a sharper toolkit, especially from the late 1970s into the biotech boom of the 2000s.
1-Tert-Butyloxycarbonyl-4,4-difluoropiperidine combines the Boc group’s reliable nitrogen protection with a piperidine ring doubly locked at the 4-position by fluorine atoms. The Boc group shields nitrogen, allowing for selective reaction sites. Double fluorine swaps at carbon-4 nudge physicochemical properties, giving the ring a mix of stability, electronic twist, and metabolic resistance that ordinary piperidines simply don’t deliver. Anyone shaping new pharmaceuticals or studying how fluorinated motifs impact biological activity runs into this compound or its derivatives. Personally, I remember days in the lab where this tool helped simplify what would have otherwise been frustrating purification steps in multistep synthesis.
The compound usually lands as a white to off-white solid or crystalline powder, with a slight but unmistakable odor typical for Boc-protected amines. Physical handling points to decent solubility in common organic solvents—think dichloromethane, acetonitrile, and methanol—which smooths out chromatographic workups. Melting point hovers between 64 and 68°C for most commercial samples. From a chemical standpoint, the Boc group brings classic acid lability; mild acids cleavage the protection, which is a practical necessity in many multi-step syntheses. The fluorinated center at 4,4 turns the piperidine notably resistant to oxidation, and dampens reactivity at those positions, which is exactly what you want in scaffold modification for drug analogues.
Lab bottles arrive with neat batch labels—CAS number 1227587-77-6, structural formula, and a minimum purity above 97%, which aligns with how quality assurance teams run standard checks by HPLC and NMR. Moisture and oxygen sensitivity limits aren’t huge issues but bottles must seal tight, and for long-term storage, a refrigerator keeps degradation in check. Anyone who’s worked with pharmaceutical-grade intermediates knows familiarity with the label speeds up shipping and inventory, and saves hours chasing paperwork during audits.
Making 1-Tert-Butyloxycarbonyl-4,4-difluoropiperidine relies on sequential logic. Start with difluorination, which builds the 4,4-fluorinated core, then bring N-protection via classic Boc anhydride in the presence of base like triethylamine. Sometimes, labs opt for the alternative: piperidine gets Boc-protected first, and then regioselective fluorination at the 4-position follows, though this path risks side products. Reaction progress gets tracked by TLC, and isolating the product usually wraps up with silica gel chromatography followed by vacuum drying. I’ve found the entire process can go from start to pure product in less than three days, given halfway decent starting material.
The Boc group lets chemists push nitrogen substitution at will, stripping protection with trifluoroacetic acid when desired. Fluorinated centers, especially at 4,4-, slow ring degradations, so folks working in peptide or small-molecule design use this for constructing analogues resistant to metabolic breakdown. Key downstream reactions include N-deprotection, alkylation, acylation, and even cross-coupling if you’ve exposed the nitrogen. To push the scaffold further, oxidative strategies target the non-fluorinated sites, while the difluoro handle can sometimes be nudged toward tertiary alcohol or amide functionalities, though yields can turn fickle. Real-world exploration of these routes happens daily in contract research organizations.
This chemical goes by a few aliases, depending on the catalog or journal—Boc-4,4-difluoropiperidine, tert-butyl 4,4-difluoropiperidine-1-carboxylate, or even CAS 1227587-77-6 in supplier lists. Major players like Sigma-Aldrich, Acros, and TCI carry the stock, each with their own product code, but a trained chemist can spot these compounds by the standard piperidine ring with Boc-protection and its twin fluorine signature on 4,4-carbons.
Diligence in handling means gloves, goggles, and working under a fume hood—especially if powders go airborne. Inhalation, contact, and accidental ingestion all trigger standard emergency procedures: wash, rinse, and seek help. MSDS sheets point out that fluorinated organics sometimes break down into acid gases if burned, so heat must stay low and wastes head into compatible disposal streams. From my own lab routines, clear labeling and buddy checks on all open bottles have never failed as basic loss-prevention. Waste containers dedicated to Boc derivatives prevent dangerous mix-ups, keeping safety in line with institutional auditing.
Medicinal chemists lean on 1-Tert-Butyloxycarbonyl-4,4-difluoropiperidine to piece together drug candidates packed with metabolic stability. The pharmaceutical industry uses such intermediates while searching for antibodies and inhibitors, especially where metabolic enzymes chew up non-fluorinated analogues. Peptide synthesis, CNS-active drug analogues, and fluorinated building block libraries all use this scaffold as a backbone. In my own collaborations, this compound saw action in optimizing lead compounds that needed just enough ring rigidity to hit protein targets but sidestep phase-I oxidation in animal models.
For every chemist tweaking molecular frameworks, the readiness of Boc-difluoropiperidine saves a ton of synthesis effort. Current R&D moves toward using selectively fluorinated piperidines as PET tracers and CNS drug precursors, where the Boc group’s straightforward removal smooths out final modifications. Universities and biotech startups patent newer modifications, targeting both higher reactivity and niche biological applications. The focus isn’t just on the compound’s own reactivity, but also how structurally tweaking the ring at the 4,4-positions unlocks whole libraries of analogues for SAR (structure–activity relationship) exploration.
Studies on fluoro-organic compounds remind us to watch out for both acute and chronic toxicity. So far, Boc-protected intermediates like this one display low direct toxicity, but final judgment demands more animal studies and careful environmental monitoring. The difluorinated positions hold metabolic stability, which means persistence if the compound ever leaks into the wild. Strict protocols on disposal, low threshold exposure limits, and close medical oversight during animal testing shape how the industry approaches larger-scale adoption. Within process labs, mild skin and eye irritation sometimes pops up, though incidences stay below serious thresholds given proper PPE and dust management.
Shifts in pharmaceutical research—toward smarter, more durable small molecules—directly benefit compounds like 1-Tert-Butyloxycarbonyl-4,4-difluoropiperidine. Predictive modeling and AI-driven synthesis now run headlong into the need for fluorinated cores that can persist through metabolic stress and cross blood–brain barriers. This compound enables biotech startups to design next-generation molecules with improved safety, structure, and selectivity, which may have been out of reach using older, less stable building blocks. As materials science looks for fluorinated piperidine derivatives to fill specialty coatings or performance additives, larger-scale synthesis and recycled feedstocks might push this once-niche compound into broader industrial and green chemistry conversations.
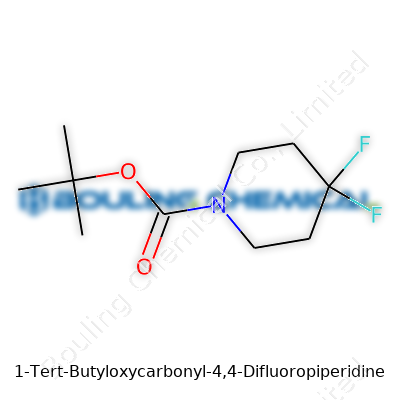
Picture a science classroom, the sharp scent of solvents, the rattle of glassware — and right in the center, a structural formula drawn on the whiteboard. 1-Tert-Butyloxycarbonyl-4,4-Difluoropiperidine isn’t simply a name pulled from a chemistry exam; it’s a compound that says a lot about modern synthetic chemistry. Here’s what brings it to life. The chemical formula is C10H17F2NO2, and the backbone is a six-membered piperidine ring. Tert-butoxycarbonyl (often written as Boc) attaches to the nitrogen at the first position, serving as a protective group. Two fluorine atoms hang off the fourth carbon — imagine two bright flags sticking out of the ring.
Anyone who’s spent time in organic labs knows that transformations rarely happen without a fight. The Boc group walks in as a sort of bodyguard. Chemists use it to protect the nitrogen while building more complex molecules, especially in pharmaceutical synthesis or peptide assembly. It survives most reactions but leaves quietly under acid, saving precious intermediates from unwanted side reactions.
Now, those two fluorine atoms at carbon four — they aren’t just window dressing. Fluorine resists many types of chemical attack and flips the switch on biological properties. Sometimes, swapping a hydrogen for fluorine ups metabolic stability or changes how the molecule binds in the body. You’ll find similar chemistry in blockbuster drugs and agrochemicals.
Drawing this out, you start with the nitrogen of the piperidine ring. Attach boc: a (CH3)3COC=O group. The fourth carbon — directly across the ring — wears two fluorines. This decoration isn’t accidental; it’s designed for a reason, and every atom plays a role.
The structure looks like this: OC(C)(C)(C)C(=O)N1CC(F)(F)CCC1. One glance at this and chemists know how to plan reactions, where to expect trouble, where potential reactivity and stability come from. In the industry, simple things like a protective group can shave months off development timelines.
Fiddling with ring systems and protective groups feels a lot like puzzle-solving. Move one piece, and the rest might fit together just right. This compound, with its Boc on nitrogen and a pair of stubborn fluorines, has been stepping into more and more syntheses lately. Whether working toward building blocks for drugs, PET tracers, or fluorinated intermediates, you often run into this backbone.
From experience, using the Boc protected form cuts headaches later in multi-step syntheses. You can keep piperidine’s lone pair out of the fray, free to focus on adding the next ring, chain, or charged group. Compare running a reaction with and without the Boc group — cleanup and yield often look completely different.
Fluorine isn’t friendly. Adding it in the right place, cleanly, strains even the most robust methods. That’s where molecules like this one come in handy — chemists can drop in a difluorinated ring exactly where they want, sidestepping harsh fluorination reagents.
The main challenge sits in scaling and cost. Not every lab can make or buy multi-gram quantities without breaking the bank. Smarter process chemistry could bring prices down, opening doors for more small labs and university groups. As more research pivots to fluorinated compounds — whether for improving drugs or making new imaging agents — access to these building blocks lets more scientists experiment and innovate.
There’s steady progress on milder, cleaner ways to install difluoro groups or to remove Boc protecting groups with fewer byproducts. Sharing methods and commercial suppliers broadening their catalogs makes a difference. It’s a balance between creative chemistry and practical business — finding safer, more efficient ways forward while making sure every lab can get the tools to build what comes next.
1-Tert-Butyloxycarbonyl-4,4-Difluoropiperidine hardly pops up in everyday conversation, but researchers in labs know its value. From where I sit, familiar with the shuffling piles of chemical catalogs on a scientist’s desk, specialty reagents like this one shape a lot of what ends up working in a pharmacy or at the clinic.
Drug discovery drives most interest in difluorinated piperidines. Medicinal chemists tinker with molecules, always hunting for ones that could block a bad enzyme or clear up an illness. Fluorine does more than just look fancy; it actually helps a drug molecule resist breakdown by the body. That’s a big deal. Drugs last longer in the bloodstream, so patients may dose less often and see results quicker.
Adding a Boc group, which is what the “Tert-Butyloxycarbonyl” part means, protects part of the molecule while chemists work on other pieces. They can remove the protecting group later, like peeling off tape after painting. This approach lets teams design drugs with far fewer unwanted byproducts. More options open up when chemical protections like Boc get used at the right steps.
This compound often shows up in the kind of research aimed at innovation. Major pharmaceutical companies sink big budgets into synthesizing new molecules. University labs, on the other hand, depend on precise intermediates while studying what minor tweaks in a lead compound might do. Having ready access to a specialty item like this lets these teams run test after test, hunting both for new therapies and for more efficient chemical steps.
For folks who never spent a weekend at a research bench, it’s easy to forget how small changes in structure — like slipping in two fluorine atoms — can turn a flop into a blockbuster drug. New medicines for anxiety, cancer, or epilepsy sometimes come from what look like minor adjustments to the amateurs’ eyes.
Pure synthesis creates headaches, especially with sensitive intermediates like these. Keeping moisture or air away means extra care, which raises production costs. Sourcing highly pure materials depends on skill, patience, and sometimes new types of equipment that can precisely control temperature and vacuum. Smaller chemical companies jump in here, offering better batch control and flexibility. Partnerships help: research centers often place custom orders for exactly the structure they want, skipping mass-market quantities entirely. Local supply chains and skilled technical support on-call give another edge.
As new therapies make headlines, specialty chemicals keep catching attention from regulatory inspectors. International guidelines push for improved tracking, safer storage, and better information sharing along the supply chain. In my own work dealing with procurement, I’ve seen spreadsheet after spreadsheet updating the latest safety data sheet or new European regulation. It can be a headache, but it’s also a steady check on quality — and reassures everyone downstream, from the scientist at the workbench to the patient waiting on results.
Years ago, it took months and a wild amount of guesswork to explore promising drug candidates. Now, researchers count on intermediates like 1-Tert-Butyloxycarbonyl-4,4-Difluoropiperidine to speed up the cycles. That means safer, better treatments reach real people sooner. Sophisticated reagents do more than fill gaps: they fuel the next wave of invention, making the path from bright idea to therapy far more direct.
Ask anyone who’s worked in a lab or on a factory floor: poor storage decisions usually come back to bite. High school chemistry taught me more about storage than about balancing equations. Someone once left a tub of sodium hydroxide next to a window, right where the morning sun heated up the glass. By lunch, the lid had buckled, powder all over the bench, and the maintenance crew wasn’t amused.
Most compounds, especially organic ones, fare better when kept in a cool, dry spot. Heat speeds up all sorts of nasty little chemical reactions. Light, particularly sunlight, can mess with molecular bonds—yellowing powders or turning liquids cloudy. Humidity adds another layer of chaos; water vapor sneaks into containers and can ruin a sample’s quality in a day. Paper packets of silica gel do their job but get soggy themselves—swap them every so often.
Labels often offer the bare minimum: “store at room temperature,” “keep tightly sealed,” or “refrigerate.” Half the time, this isn’t enough. Imagine carrying an ice pack to your lab every morning, but the warning only said to ‘avoid heat’—leaves a lot open to interpretation. Some people think ‘room temperature’ means 30°C in summer. Usually, that’s too warm for comfort, let alone for a sensitive chemical.
Experience shows that if the stuff wasn’t expensive in the first place, the real cost hits when it spoils early. It never hurts to ask around—call the supplier, read the manufacturer’s full safety sheet, and dig through forums for tricks. An old-timer at my first job insisted on double-bagging moisture-sensitive powders in thick ziplocks. The habit stuck. Simple habits like writing the ‘opened on’ date can save headaches later, especially if the shelf life is fuzzy.
If you’re looking after an acid or solvent, keep it far from anything alkaline—corrosive accidents cause more trouble than a cluttered bench ever did. Flammable substances belong in a fireproof cabinet, not tucked next to the printer or an exposed electrical outlet. I’ve seen printers spark, spilling toner into the nearest box. Thankfully, it was only paperwork—imagine if it had been a canister of ether.
Try not to mix work with clutter. Chemicals deserve real shelves and locked cabinets. Put similar hazards together; don’t toss bottles onto an already crowded rail. Invest in labels with bold colors and clear writing—faded marker ink fools no one at 7 a.m., and if the power goes out, readable labels really help.
Training matters more than laminated checklists. Get everyone in the habit of checking for leaks, odd smells, or clumped powders. If something feels off, act fast. Regular inspections cut down on surprises, and a simple inventory avoids those “Where did we put that corrosive last week?” moments.
Some folks balk at moving chemicals into a well-ventilated space because it ‘takes too much time.’ The minute saved isn’t worth the disaster that can follow. If you can’t get a vented store room, at least open some windows. Invest in good containers. Cheap lids leak, sometimes invisibly. Nobody needs the stress of cleaning up a spill because of a cracked cap.
Looking after chemicals isn’t about ticking boxes; it’s about making smart choices every day. A minute now keeps your lab and your nerves intact tomorrow.
Open up a chemical catalog, scroll long enough, and chemicals with long names pop up everywhere. 1-Tert-Butyloxycarbonyl-4,4-Difluoropiperidine isn’t a household name, but chemists know it as a clever piece for building more complex molecules. If you’re eyeing this compound for research or synthesis in the lab, it’s worth remembering that the safety routines you skip today can return as problems tomorrow.
The faint smell of something sweet or sharp in the air, a drop of unknown liquid on your gloves — stories in chemistry labs don’t always have happy endings. This compound comes with its own set of risks, thanks to those difluorinated rings and the bulky protecting group. It doesn’t announce itself as dangerous with fumes, like some others do, but don’t let that fool you. Many organic compounds, especially those with fluorine or carbonyl groups, have a knack for getting under your skin, irritating eyes, or making trouble if you breathe in dust or vapors.
Grabbing a bottle of 1-Tert-Butyloxycarbonyl-4,4-Difluoropiperidine means goggles go on, gloves go on, and that bottle doesn’t leave the fume hood unless you have a solid reason. Some of us have cut corners and paid for it with rashes or headaches. Even a small spill can add up over a day, especially if you’re handling several grams. Pipetting or weighing small volumes makes it tempting to take shortcuts, but chemicals like this tend to stick to gloves, and that transfers to doorknobs and laptops. A good lab coat does more than protect your shirt; it reminds you to think about what you’re touching throughout your day.
Moisture and sunlight aren’t friends to Boc-protected compounds. Storing this one in a dry, cool spot – tightly sealed – keeps it usable longer. That’s not just about saving money. Decomposition products can surprise you, and sometimes what grows in a forgotten bottle delivers bigger headaches than the original stuff. If you notice the color shifting, or smell that tells you things have changed, move it out of common areas and report what you see.
Skin contact and inhalation count as the main ways people get exposed. Organics with fluorine like to sneak in through skin, and many folks think a splash on a glove is no big deal. Hydrophobic solvents and lipophilic molecules get through simple nitrile gloves faster than you expect. Regularly changing gloves avoids a lot of problems.
Disposing of leftover material or contaminated glassware means treating everything as hazardous waste. Dumping things down the sink might feel like it’ll disappear, but that’s asking for contamination or an environmental citation. I’ve seen labs get warnings for washing down less hazardous materials—no one wants a more serious problem. Absorb small spills with vermiculite or paper towels, bag it, and send it out with the waste stream the right way.
Basic habits like labeling spent containers, recording incidents as they happen, and checking safety data sheets help everyone stay out of the ER. Too many accidents trace back to someone skipping these small steps. Running regular refresher training, keeping emergency showers unblocked, and creating a culture where people actually speak up about mistakes make a world of difference.
It only took one bad incident during grad school with an unmarked bottle for me to stop trusting my memory alone. Double-check the label, keep detailed notes, and never assume anyone else left things safe—this mindset paid off more times than I can count. Working with 1-Tert-Butyloxycarbonyl-4,4-Difluoropiperidine reminds me that new reagents don’t mean new rules—just more reason to stick to what works for protecting both people and the work itself.
Ask any buyer what they care about, and you’ll usually get two questions right away: how pure is this stuff, and how can I get it? These aren’t esoteric concerns. They influence everything, down to safety rules in a factory and whether or not a small business survives long term.
Lots of folks glaze over when purity percentages are mentioned, like “99.9%—good enough, right?” That extra decimal might seem like window dressing, but it changes everything for people who deal with sensitive processes or strict regulations. I used to work alongside a team making industrial cleaners. Whenever the purity dropped below our spec, reactions fell apart or gave weird byproducts. Sometimes you’d catch it from a weird smell, but businesses can't rely on lucky noses.
Too much impurity, and you risk costly recalls, hazardous fumes, or worse. A few years back in the food world, a batch with the wrong specifications ended up shutting down a whole production line. The cleanup took months, and the costs ballooned. That’s just one example—I’ve heard engineers joke that “purity is peace of mind,” but there’s no joke about the consequences when it’s ignored.
On the face of it, packaging might seem like a backroom detail. But the size options set the limits for everyone who buys or resells the product. Think about a local business. Maybe they don’t run 24/7, so they don’t want pallets crowding their back hallways. I worked with a soapmaker who paid a premium for smaller drums just to fit them in her tiny workshop.
For high-volume movers, large bags or bulk tankers keep costs down and reduce the amount of plastic or cardboard waste that piles up. But for researchers or hobbyists, anything above a small bottle collection is just overkill. If the company offers only 25 kg sacks and nothing else, anyone not meeting that scale is forced to turn elsewhere or risk spoiling raw materials through slow use.
Offering a variety of sizes keeps everyone happy—from the multinational factory down to a high school science club. It’s not just convenience; some packaging keeps the product stable better than others. Light-sensitive powders need dark bottles. Large drums can dent or let in air, messing up the whole batch.
In the last few years, shortages have driven people to seek backup suppliers. Those who survived the crunch were the ones with several options—big boxes for the companies, small bottles for the rest. Some even offered recycling programs so empty drums wouldn’t clutter up warehouses or landfills.
The practical answer—ask for what you need, and don’t assume new suppliers have you covered. Companies thrive by listening, so sharing feedback leads to more choices. Regulations keep changing, and companies adjust, but everyday buyers influence those moves by speaking up. Even a small switch, like adding a 1-kg pouch for niche buyers or spurring cleaner packaging material, grows from conversations like these.
Down the line, the best outcome means both purity and packaging get treated as frontline issues, not afterthoughts. That benefits everyone using, storing, or selling the product, turning simple questions into better products and smoother business for all.
| Names | |
| Preferred IUPAC name | 1-(tert-Butoxycarbonyl)-4,4-difluoropiperidine |
| Other names |
Boc-4,4-difluoropiperidine tert-Butyl 4,4-difluoropiperidine-1-carboxylate tert-Butyloxycarbonyl-4,4-difluoropiperidine N-Boc-4,4-difluoropiperidine |
| Pronunciation | /ˈwʌn tɜːt ˌbjuːtɪlˌɒksiˌkɑːˈbɒnɪl ˌfɔːrˈfɔːˈdɪˈflʊəˌpaɪpəˈriːdiːn/ |
| Identifiers | |
| CAS Number | 861155-88-0 |
| 3D model (JSmol) | `JSmol('CC(C)(C)OC(=O)N1CC(CC1)(F)F')` |
| Beilstein Reference | 3838735 |
| ChEBI | CHEBI:189353 |
| ChEMBL | CHEMBL3702062 |
| ChemSpider | 178159 |
| DrugBank | DB08337 |
| ECHA InfoCard | 03a74f10-98c7-41e7-a03f-c18738e48c01 |
| EC Number | EC 700-130-8 |
| Gmelin Reference | 1971085 |
| KEGG | C16518 |
| MeSH | D000072650 |
| PubChem CID | 75105015 |
| RTECS number | TI4020000 |
| UNII | 904919N85Q |
| UN number | UN3272 |
| Properties | |
| Chemical formula | C10H17F2NO2 |
| Molar mass | 209.23 g/mol |
| Appearance | White solid |
| Odor | Odorless |
| Density | 1.17 g/cm3 |
| Solubility in water | Slightly soluble |
| log P | 1.36 |
| Vapor pressure | 0.5 mmHg (25 °C) |
| Acidity (pKa) | pKa = 11.7 |
| Basicity (pKb) | pKb = 5.20 |
| Magnetic susceptibility (χ) | -73.2e-6 cm³/mol |
| Refractive index (nD) | 1.390 |
| Dipole moment | 2.97 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 416.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -749.6 kJ/mol |
| Pharmacology | |
| ATC code | This product does not have an ATC code. |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302 + H312 + H332: Harmful if swallowed, in contact with skin or if inhaled. |
| Precautionary statements | P210, P261, P280, P305+P351+P338, P337+P313, P370+P378 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | > 95 °C |
| NIOSH | NA PR0285000 |
| REL (Recommended) | 1.01 to 1.50 |
| Related compounds | |
| Related compounds |
Piperidine 4,4-Difluoropiperidine 1-Boc-piperidine 1-Tert-Butyloxycarbonylpiperidine N-Boc-4,4-difluoropiperidine N-Boc-piperidine-4-one Piperidine-4-carboxylic acid |