1-Propylpyrrolidine has lived a quiet life behind the scenes, compared to other well-known heterocyclic compounds. Early chemistry books from the first half of the 20th century mention its structure in the context of nitrogen-containing rings. It's not a molecule that grabbed headlines, yet synthetic organic chemists have worked with it for decades. The backbone of pyrrolidine rings often appears in pharma research, with substitutions like the propyl group leading to a new array of molecules with slightly different profiles in terms of both reactivity and function. Experience from working in university labs tells me that the rise of automation in chemical synthesis and analytics during the 1990s gave this class of molecules a better chance to surface in drug screening and materials development.
1-Propylpyrrolidine doesn’t turn up in household goods, but chemical suppliers keep it on their lists for research and specialty manufacturing. This compound fits into projects needing specific saturated nitrogen rings. Researchers in medicinal chemistry and specialized industrial synthesis often pick up 1-Propylpyrrolidine for its unique blend of stability and reactivity in certain reactions. Usually sold as a colorless or pale liquid, it's not flashy, and those who value it tend to work on small-scale specialty chemistry projects or as intermediates in making more elaborate products.
As a five-membered ring with one nitrogen, 1-Propylpyrrolidine holds a boiling point that usually settles just above 140°C. With a molecular weight somewhere near 127 g/mol, it flows readily at room temperature. It smells a bit fishy—like many amine-containing compounds do. Its solubility in water is limited, but it mixes well with organic solvents such as ether, chloroform, and alcohols. The structure gives it increased basicity compared to more neutral molecules, owing to the lone pair on the nitrogen atom. Chemists often leverage this basicity in acid-base reactions or as a ligand in coordination chemistry.
Most vials or drums containing 1-Propylpyrrolidine carry CAS number 23245-48-7, making searches easier for lab purchasing teams. Labels highlight its purity, with top suppliers pushing for levels above 98%, sometimes showing details about water or residual solvent content. UN numbers for hazardous transport may apply depending on national rules. Safety data sheets repeat warnings about inhalation and direct contact. In my experience, technical datasheets lay out compatibility, storage advice (cool, dry, away from oxidizers), and shelf life—usually measured in years if unopened and properly stored.
The lab approach tends to start with pyrrolidine itself, a simple five-membered ring with one nitrogen atom. Alkylation of pyrrolidine with 1-bromopropane, under controlled temperature and in the presence of a strong base, leads directly to 1-Propylpyrrolidine. Solvents like acetonitrile often support the reaction, and purification can take time since unreacted starting material can linger in the product. For industrial use, scale-up involves similar chemistry, only with more robust safety mechanisms and larger vessels, since pressurized reactors help accelerate the process. Steam distillation or careful distillation under reduced pressure brings out a clean batch.
1-Propylpyrrolidine stands out for its stability but also has just enough nucleophilicity to participate in addition and substitution reactions. The nitrogen often takes part in alkylation, acylation, and oxidation steps, serving as a base or a nucleophile. When paired with reactive acid chlorides or anhydrides, acyl derivatives emerge. Hydrogen abstraction under strong conditions can tweak the propyl side chain, leading to both saturated and unsaturated derivatives. Given the right oxidant, the nitrogen gets pushed up to an N-oxide, which alters the molecule's properties even further. Chemical modifications often underpin the development of analogs for pharmaceutical screening.
Besides the systematic name, you might run into "N-Propylpyrrolidine" or "1-N-Propylpyrrolidine" on chemical inventories and supplier catalogues. These names mean the same molecule, but some suppliers add branding or catalog codes. Within research papers, writers save space with short-hand versions, but chemical databases stick with the IUPAC name and the CAS number to prevent confusion. Some overseas manufacturers use local or trade-specific names, so referencing the CAS number eliminates doubt.
No matter the size of your lab, contact with 1-Propylpyrrolidine feels the same as any low-molecular-weight amine—pungent and potentially irritating. Direct eye or skin exposure burns. Inhalation of vapors creates discomfort in the mucous membranes and can cause dizziness if used in poorly ventilated spaces. Fume hoods or effective local exhaust keep the air clean. Nitrile gloves, chemical splash goggles, and lab coats serve as basic gear. Spill control comes down to standard absorbent materials and disposal in line with local hazardous waste rules. Many countries now require a risk assessment with all amines thanks to their environmental impact.
Pharmaceutical synthesis offers the richest domain for 1-Propylpyrrolidine. Process chemists sometimes incorporate it as an intermediate during routes to more elaborate bioactive molecules. It's also valuable as a base or building block for the synthesis of drug candidates containing the pyrrolidine scaffold—a motif that's common in enzyme inhibitors and CNS-targeted agents. Some niche areas of catalysis have tested its use as a ligand for transition metals in asymmetric synthesis. On the industrial front, small amine-based molecules like this one occasionally rise up in fine chemical manufacturing, especially as transient species in formulation chemistry. Laboratory research stays the main consumer, given the relatively low annual demand.
In both academia and industry, there's regular curiosity about tuning the properties of the pyrrolidine core with different substituents. Medicinal chemists hunt for molecules that boost bioavailability or specificity by making small changes, and 1-Propylpyrrolidine joins libraries of similar rings to screen for activity. In my time working in medicinal chemistry, I saw it used to adjust the lipophilicity or basicity of lead compounds. Analytical researchers also probe its behavior using chromatography and spectroscopy to build databases of physical and chemical properties for future rapid identification. Other projects experiment with integrated computer modeling to extrapolate its potential as a pharmacophore or as part of a supramolecular assembly.
Data remains limited, but small ring amines warrant caution due to their reactivity and ability to pass through biological membranes. Acute toxicity in rodents indicates moderate lethality at high doses, with symptoms centering on CNS depression and respiratory issues. Human exposure data is scarce, so regulatory agencies assign it risk phrases based on structural similarity to other cyclic amines, which call for assuming moderate hazard in the absence of direct evidence. Research animals show symptoms consistent with amine exposure: agitation, incoordination, salivation. Chronic toxicity studies lag behind, but anecdotal reports from industrial chemists suggest prudent handling to avoid cumulative exposure, given possible liver and kidney stress.
As chemical industries lean further into custom synthesis and smaller scale, high-value manufacturing, 1-Propylpyrrolidine stands a fair chance of wider application in making targeted pharmaceuticals and fine chemicals. Computational drug design models keep flagging substituted pyrrolidines as motifs worth exploring for mental health and rare disease therapies. Green chemistry is another area poised for growth—future synthetic routes will likely cut waste by rethinking alkylation reagents or using bio-based feedstocks. With regulatory agencies demanding cleaner data and validation, work on toxicology and workplace safety will expand. Chemists chase efficiency and sustainability, so 1-Propylpyrrolidine has its part to play, even if quietly, in the years ahead.
Outside of chemistry textbooks, not many folks chat about 1-Propylpyrrolidine over dinner. Still, this clear, oily liquid finds its way into a surprising number of industries. It isn’t something you see on a store shelf. Most people don’t realize that its real job is backstage, supporting larger systems that many rely on without even thinking about chemicals at all.
Pharmaceutical research craves building blocks like 1-Propylpyrrolidine. Chemists use it as a starting place—or sometimes as a stepping stone—in creating drugs. For example, certain antidepressants and newer experimental treatments for neurological disorders have roots in molecules shaped by 1-Propylpyrrolidine. The compound helps scientists manipulate the structure of medicines, testing new versions with slightly tweaked properties to find the one that shows real results. It saves money and time in labs, which means researchers can move faster in the race to tackle tough diseases.
The story doesn’t end in the world of medicine. Factories looking to design specialty chemicals will use 1-Propylpyrrolidine as a handy reagent. Take agricultural products, for instance—crop protection depends on precise molecular shapes. 1-Propylpyrrolidine helps create the right fit for these molecules, letting them do their job in battling pests without causing too much trouble elsewhere. There’s no magic; it’s just chemistry doing the heavy lifting so that farmers can grow more reliable crops.
There’s a practical side too. In my time working near a coatings lab, I saw technicians reach for chemicals like this as solvents or supporting ingredients in reaction vessels. Sometimes, chemists speed up reactions by introducing nitrogen-based helpers like 1-Propylpyrrolidine. This chemical’s small structure and flexible carbon chain mean it can dissolve or transport all kinds of materials that would otherwise be stubborn. Solvents aren’t just for thinning paint—they’re the secret sauce behind smooth production lines in everything from plastics to electronics.
Working with chemicals brings up big questions about safety and the environment. 1-Propylpyrrolidine, like other amines, can be hazardous when handled carelessly. It’s not toxic at the smallest whiff, but spills and fumes create real headaches for anyone stuck cleaning up a lab or factory. The chemical industry works to tighten up handling practices. For people outside the lab, stricter labeling and improved training can go a long way in preventing accidents and exposure. Pushing for green chemistry means designing processes that either use safer chemicals or make sure chemicals like this one don’t hang around in wastewater or air vents.
Some researchers have started investigating what 1-Propylpyrrolidine can offer in newer technologies. Engineers have tried it in the hunt for improved battery electrolytes or as part of chemical sensors. These are early days, but every time a new application comes up, someone has to re-examine how to use this tool more safely and efficiently. In research or industry, one small molecule can unlock a surprising amount of potential, and 1-Propylpyrrolidine is no exception.
1-Propylpyrrolidine doesn’t pop up in casual conversation unless you spend a lot of time in a laboratory. I’ve come across plenty of molecules in textbooks and real life that sound almost identical but do completely different things. With 1-Propylpyrrolidine, the structure speaks to a class of compounds chemists bump into when exploring organic synthesis, the world of pharmaceuticals, or even specialty chemicals.
The chemical formula lands as C7H15N. That’s seven carbons, fifteen hydrogens, and a nitrogen—the backbone of a lot of everyday organic chemistry. The way it’s built, you’ve got a pyrrolidine ring, which is just a five-membered, saturated nitrogen ring, with a propyl group tagging along at the first carbon. Drawing it out, you see how the addition changes its behavior compared to straight pyrrolidine. Looking up the molecular weight means adding up the atomic weights: carbon gives roughly 12.01, hydrogen adds 1.008, and nitrogen brings about 14.01. Running the math, the total weight for 1-Propylpyrrolidine lands at around 113.20 grams per mole.
I used to gloss over molecules like this when I was flipping through compound lists for class. But doing lab work and talking with process chemists, I learned how much a slight structural change can impact the way a molecule acts. You take a pyrrolidine ring and stick on a propyl group—suddenly you’re looking at a new landscape for reactivity, solubility, stability, maybe even toxicity.
1-Propylpyrrolidine isn’t a blockbuster drug or star monomer, but structures like this represent the hundreds of building blocks that make up the workflow in synthetic chemistry. If you’ve ever wondered why researchers spin their wheels testing all kinds of similar-looking molecules, it’s because tweaking a molecule this way can create compounds with entirely new properties, sometimes opening doors in drug discovery or materials science. I’ve seen teams go from a common starting structure, like the humble pyrrolidine, and through small steps turn it into something with real, practical value.
One challenge working with smaller organics like 1-Propylpyrrolidine involves safety and handling. Nitrogen-containing rings aren’t always forgiving. Some versions can have funky smells, cause irritation, or volatilize quickly. Anyone diving into organic synthesis knows the importance of a good fume hood, gloves, and double-checking material safety data before even considering opening a bottle. Years back, a lab mate opened a related cyclic amine without gloves—ended up regretting it thanks to the skin absorption and persistent stink. Respect for these compounds comes fast after even one misstep.
Not every small molecule is destined for the pharmacy shelf, but 1-Propylpyrrolidine and compounds like it help expand the research toolkit. They offer new starting points or intermediates for drug design, or might eventually help craft better solvents or specialty chemicals. I’ve seen colleagues use molecules like this as scaffolds for creating complex libraries to test against bacterial proteins or neurological targets. Even if only a fraction get used in a final product, exploratory work grows our understanding of structure-activity relationships.
Regulation and documentation remain crucial. Tracking the impact of compounds, waste streams, and potential hazards keeps science moving forward without piling up risk. In my experience, regular audits and peer check-ins make a big difference—no shortcuts worth the fallout from a safety lapse.
Details like a simple chemical formula or molecular weight hold more than trivia; they open up conversations about why even a subtle change in structure can matter. Whether you’re just starting out with chemistry or making a career of it, every molecule teaches something new about the world’s hidden networks.
Plenty of folks see long chemical names and zone out, but handling something like 1-Propylpyrrolidine hits close to home for anyone who’s ever stepped into a lab, warehouse, or even a backroom stockpile at a small business. I’ve seen careless storage choices lead to spills, leaks, and headaches for everyone involved. The rules written on safety sheets only matter if people on the ground respect them.
This compound falls under the umbrella of amines. It’s colorless, which fools the eye, but the smell reminds you it doesn’t play nice. 1-Propylpyrrolidine reacts strongly with acids and oxidizers, so tossing it on a shelf with random containers raises real risks. I've watched coworkers scramble after a container dripped on a cardboard box—a mess that could’ve been avoided with better habits. Keeping the workplace safe means thinking one step ahead, not just filing away MSDS sheets and calling it a day.
Forget fancy systems—solid, repeatable habits work best. Shelves should always keep this chemical off direct sunlight and away from any heat sources. Metal cabinets provide extra peace of mind, especially with proper ventilation in the storage area. Temperature control matters. Swinging between hot and cold stresses the container, and nobody enjoys patching leaks mid-shift.
Dry areas trump damp corners every time. Water leads to corrosion, and nothing good follows from rusty containers. I once had to help clear a storage space in a humid basement where water dripped from pipes overhead. Sure enough, labels blurred, caps rusted, and everyone felt nervous about what they might grab.
Chemicals stay safest in their original containers, but sometimes repackaging happens. Only use containers built for strong solvents, with clear, legible labeling that covers the chemical name, opening date, and any handling dangers. It’s tempting to stuff leftovers in easy-to-find bottles—old soda or water bottles have no place here. I’ve heard of a time someone mistook a chemical for a soft drink. No one wants that story repeated.
This stuff acts up near acid, oxidizers, and strong bases. Separate storage cuts risk, and a locker just for amines pays off in lower stress over the long haul. I learned early: always check the shelf before adding new stock. Lazy stacking invites disaster.
Sensible storage pairs well with spill kits nearby—not jammed in a locked office. Absorbent pads, gloves, and goggles should always be within arm’s reach. In one outdated facility I worked in, hunting down these supplies cost minutes we didn’t have during a leak. Emergency numbers belong at eye level, not buried under piles of paper.
People lose track. Chemicals disappear behind boxes of printer paper or outdated equipment if nobody checks monthly. Regular inspections keep records honest, catch early leaks, and remind everyone why careful storage matters, not just to management, but to themselves and their families.
The best approach to storing 1-Propylpyrrolidine looks simple from the outside, but those little details protect wallets, health, and peace of mind. Every job gets easier—and safer—when folks pay attention at the shelf, not just at the desk.
1-Propylpyrrolidine turns up in labs more often than you might expect, especially in chemical synthesis work and drug development. It’s one of those compounds with a structure that looks like it walked out of a college organic chemistry class—compact and slightly intimidating. The question that comes up is whether 1-Propylpyrrolidine is safe for people working with it, or if there are risks that get overlooked in day-to-day lab routines.
My first real job in research involved a bench with endless glassware and odd-smelling liquids, many of which didn’t come with clear safety labels. It’s easy to get into bad habits around chemicals the longer you stay in the lab. I remember working with similar pyrrolidine derivatives. The safety data sheets (SDS) acted as our go-to, and the advice there rarely minced words—respiratory irritation, potential organ effects, skin or eye burns. If you’ve handled any amine compounds, you learn to respect the warnings: gloves, goggles, fume hood. Even the smallest spills can leave you with a headache or a chemical taste in your mouth if you drop your guard.
Direct toxicity data on 1-Propylpyrrolidine can be tough to hunt down. Few compiled safety studies zero in on it, but its close cousins all show a similar story—potential for skin and respiratory irritation, central nervous system effects if inhaled or absorbed through the skin, and risk for organ problems after repeated exposure. Many solvents and amine-based chemicals fall into that bucket.
Material safety data from chemical suppliers highlight the need for protective gear and ventilation. Toxicology references suggest a risk for moderate to severe effects if someone inhales concentrated vapor or gets enough on their skin. Nobody designs experiments expecting accidents, yet chemical exposure can creep in with a cracked glove or a lab coat that’s a day overdue for washing.
Colleagues who’ve spent years around amine-like chemicals mention headaches, skin irritation, and even sensitization—where the body overreacts with repeated contact. An accidental splash or a fume escaping from an open bottle can bring on coughing fits or eye burning that lingers for hours. I once scrubbed my hands raw after a spill, convinced that if the smell stuck around, so could the effects.
Longer-term health risks matter, too. Studies with related compounds hint at possible liver and kidney stress after repeat exposure. While there isn’t conclusive evidence for every kind of amine, enough red flags pop up in animal studies to treat these compounds with care.
Good habits solve problems before they start. Proper PPE goes a long way—nitrile gloves, eye protection, and solid ventilation or fume hoods cut risk way down. Sharpening training and making sure everyone reads the safety sheets means no one gets caught off guard. Not every lab has a shower or eyewash just around the corner, but everyone should know where they are and how to use them.
Getting management to invest in safer handling doesn’t always come easily. In tight-budget environments, basics like fume extractors or regular PPE supplies get skipped. The fix often comes with persistence—crew members raising concerns and sharing stories about close calls, insisting that shortcuts aren’t worth a hospital trip. Safer substitutes might not always exist, but investing in training and simple precautions often costs less than dealing with a workplace injury.
Chemistry keeps shifting, pulling in new compounds and risks along the way. With 1-Propylpyrrolidine, the answer stays straightforward: respect its hazards, use common sense protection, and push for safer setups whenever possible. In research, habits make more difference than any technical fix, and keeping an eye open for better practices helps everyone walk out of the lab healthy at the end of the day.
Ask any organic chemist about synthesizing 1-propylpyrrolidine, and you'll probably get a small grin and a story or two. This compound turns up in plenty of R&D projects, including pharmaceuticals and advanced materials. Despite its simple appearance—a pyrrolidine with a propyl group stuck on—the ways folks make it tell you quite a bit about which lab you’re sitting in and what tools prove handy.
The classic route leans on a straightforward principle: start with pyrrolidine and tack on a propyl group. The favored method, N-alkylation, calls for mixing pyrrolidine with a propyl halide—usually something like 1-bromopropane—in a base such as potassium carbonate. This reaction gets a lot done in a simple glass flask. Pull out the product with a round or two of extraction, toss in a purification step with distillation or chromatography, and you’re in business.
This all-in approach, especially with accessible reagents, means it’s popular both at benchtops in universities and pilot plants in industry. One real sticking point with this method involves over-alkylation—when you get more propyl groups slapped on than you want, leaving you with a mix of products. Careful control of ratios and temperature usually keeps things on track.
Chemists love options. Reductive amination gives them another tool. Starting out with pyrrolidine and a propanal (sometimes even propionaldehyde), the process relies on forming an imine or iminium intermediate. Then, by tossing in a reducing agent, you drive things straight to the secondary amine. A favorite reducing agent is sodium triacetoxyborohydride, but hydrogen over a metal catalyst works too if purity matters a lot. This method keeps side reactions to a minimum and cuts down on over-alkylation, but it does ask for a bit more finesse when it comes to setting up the conditions.
I’ll tell you from experience: Sometimes the choice between N-alkylation and reductive amination boils down to what’s sitting in the chemical cabinet and how much time everyone has. In smaller-scale syntheses or if someone wants a quick proof-of-concept, N-alkylation takes the win. As things scale, concerns about purity and yield start tipping the decision toward reductive amination.
Green chemistry keeps nudging folks toward smarter, less wasteful syntheses. Solvent-free conditions, microwave-assisted synthesis, or even alternative energy sources pop up in the literature. These methods promise shorter reaction times and cleaner product, which make chemists happy and plant managers breathe easier about waste disposal. But ask around, and many will admit that old habits, budget constraints, and availability of equipment slow the switch.
If labs want better yields and cleaner processes, they need to lean harder into greener solvents and maybe even enzymatic approaches down the line. Partnerships with companies working on renewable reagents could tip the balance. Training young chemists to see beyond just yields and cost, and nudging them to think about sustainability from the start, could shift the needle for future syntheses of compounds like 1-propylpyrrolidine.
Science may move slower than some like, but that extra bit of thought—on reagents, on safety, on impact—often helps more in the long run than cutting corners ever does. And in my book, that’s worth the extra time at the bench.
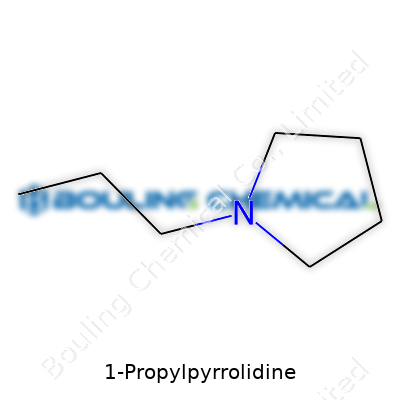
| Names | |
| Preferred IUPAC name | N-propylpyrrolidine |
| Other names |
N-Propylpyrrolidine 1-Propyl-pyrrolidine |
| Pronunciation | /waɪn-ˈprɒpɪl-pɪˈrɒlɪdiːn/ |
| Identifiers | |
| CAS Number | 104-68-7 |
| Beilstein Reference | 0721351 |
| ChEBI | CHEBI:18943 |
| ChEMBL | CHEMBL3207586 |
| ChemSpider | 59589 |
| DrugBank | DB02177 |
| ECHA InfoCard | ECHA InfoCard: 100_010_395 |
| EC Number | 216-053-4 |
| Gmelin Reference | 8990 |
| KEGG | C18953 |
| MeSH | D017721 |
| PubChem CID | 13880 |
| RTECS number | UQ8925000 |
| UNII | 999420JD4B |
| UN number | UN3276 |
| CompTox Dashboard (EPA) | DTXSID9039399 |
| Properties | |
| Chemical formula | C7H15N |
| Molar mass | 99.186 g/mol |
| Appearance | Colorless liquid |
| Odor | amine-like |
| Density | 0.867 g/mL at 25 °C (lit.) |
| Solubility in water | Soluble |
| log P | 0.97 |
| Vapor pressure | 2.93E+01 mmHg at 25 °C |
| Acidity (pKa) | 11.27 |
| Basicity (pKb) | 4.16 |
| Magnetic susceptibility (χ) | -7.42×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.426 |
| Viscosity | 0.78 mPa·s (25 °C) |
| Dipole moment | 1.25 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 360.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -30.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4433.2 kJ/mol |
| Pharmacology | |
| ATC code | No ATC code |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | {"GHS07"} |
| Signal word | Warning |
| Hazard statements | H226, H302, H312, H315, H319, H332 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P280, P303+P361+P353, P370+P378 |
| Flash point | 61 °C (142 °F; 334 K) |
| Autoignition temperature | 215 °C |
| Explosive limits | Explosive limits: 1.2–10.6% |
| Lethal dose or concentration | LD50 (oral, rat): 252 mg/kg |
| LD50 (median dose) | LD50 (median dose) of 1-Propylpyrrolidine: "400 mg/kg (rat, oral) |
| PEL (Permissible) | Not established |
| REL (Recommended) | 64 mg/L |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
N-Methylpyrrolidine N-Ethylpyrrolidine N-Propylpiperidine Pyrrolidine 1-Butylpyrrolidine |