Chemists pushed the boundaries of heterocyclic chemistry in the late 20th century, looking for functionalized pyrroles that could pave the way toward new medicines and materials. The arrival of 1-Phenylsulfonylpyrrole opened a fresh chapter. Before its development, pyrrole chemistry had already earned a reputation for bioactivity and electronic versatility, but there was a problem—stability and controlled reactivity often fell short. Sulfonyl protection, with its knack for tuning electronic properties, brought new options. I remember early articles describing phenylsulfonyl groups in pyrrole chemistry; optimism rose quickly. The compound’s synthesis wasn't an accident, but a targeted effort shaped by a need for more robust platforms for cross-coupling and streamlined functional group exchange. Over time, the chemistry bench saw 1-phenylsulfonylpyrrole shift from an oddity to a tool with recognized utility in labs across the world.
1-Phenylsulfonylpyrrole stands out in any catalog of heterocyclic intermediates. Chemically, researchers see a simple five-membered pyrrole ring, but the presence of a bulky phenylsulfonyl group attached to the nitrogen flips the script—the molecule manages both protection and activation. The sulfonyl group blocks reactive nitrogen sites, allowing for smooth handling in multistep synthesis. In the lab, it arrives as a white or pale crystalline solid, stable on the shelf and straightforward to weigh and handle, avoiding the fussy sensitivity many pyrroles are known for. Chemists know it for resilience, and it quietly acts as a protector in more than a few reaction sequences for pharmaceutical and organic electronics research.
Solid at room temperature, 1-Phenylsulfonylpyrrole often appears as a fine, crystalline powder. The melting point sits comfortably above 120°C, reflecting hearty intermolecular forces from its aromatic bulk and sulfonyl functionality. Solubility favors polar aprotic solvents such as DMF, DMSO, or acetonitrile, yet it resists casual dissolution in nonpolar organics, a feature some see as a handling benefit. In IR spectra, strong peaks in the 1300–1150 cm-1 range underline the sulfonyl group, while 1H NMR confirms the pyrrole backbone with characteristic chemical shifts alongside phenyl resonances. Its chemical stability under standard conditions makes storage less of a headache—but its reactivity blossoms only under purposeful conditions, usually involving base or transition metal catalysis.
Manufacturers typically ship 1-Phenylsulfonylpyrrole in moisture- and light-protected containers, marked with clear labeling that lists the batch number, purity (often over 98%), and storage guidelines, commonly under ambient or mild refrigeration. Safety pictograms warn about skin and eye irritation—personal experience reminds me not to skip gloves when weighing the powder. Detailed MSDS documents outline transport and disposal, since the compound, like other sulfonyl derivatives, isn't eco-friendly if poured down the drain. Chemical suppliers often provide full HPLC, NMR, and IR spectra on request, ensuring researchers can troubleshoot any strange results without guesswork.
The synthetic pathway for 1-Phenylsulfonylpyrrole usually leans on two routes. One takes pyrrole directly, reacting it with benzenesulfonyl chloride in the presence of a mild base such as triethylamine or aqueous sodium carbonate. This process proceeds in solvents like dichloromethane or toluene, offering reasonable yields after purification. Another workflow, useful when selectivity matters, involves stepwise protection—starting from an N-unsubstituted pyrrole, a chemist introduces the phenylsulfonyl group under mild conditions, sometimes chilling the mixture to control overreaction. Recrystallization from ethyl acetate or hexanes gives a workable solid, which, in my experience, usually arrives with clean analytical signatures. Labs focusing on scalability streamline by leveraging batch reactors and constant monitoring of pH and temperature: small changes here can make a significant difference between gram and kilo scales.
1-Phenylsulfonylpyrrole’s charm lies in selective reactivity. Protected at the nitrogen, it behaves beautifully in metal-catalyzed coupling reactions—chief among them, the Suzuki and Stille routes for attaching complex aromatic groups at strategic ring positions. Nucleophilic substitution at the 2- and 3-positions draws on the sulfonyl group’s electron-withdrawing power, steering electrophiles toward the ring. After these steps, deprotection with sodium amalgam or reductive protocols removes the sulfonyl group, freeing up the nitrogen for further elaboration. Chemists often use the phenylsulfonyl tag as both a “mask” and an activator in domino sequences, saving time and reducing purification headaches. In hands-on practice, I’ve watched this approach transform simple precursors into pyrrole-containing molecules tough to reach using other protecting groups, laying foundations for natural products and advanced materials.
Across catalogs and research papers, 1-Phenylsulfonylpyrrole goes by several names. You’ll see N-Phenylsulfonylpyrrole, 1-p-Toluenesulfonylpyrrole (in cases where the phenyl carries methyl groups), and sometimes simply as NSO2Ph-pyrrole. Less common designations include pyrrole, 1-(phenylsulfonyl)- or “PhSO2-pyrrole.” Chemical suppliers and publications still list synonyms, hoping to unmask any confusion from name variants in patents or procurement software. Tracking synonyms helps untangle literature searches and ensures the right compound hits the bench, not an under-protected analogue.
Handle 1-Phenylsulfonylpyrrole with the same respect you’d give most sulfonyl reagents. The phenylsulfonyl group doesn’t have the volatility of alkyl sulfonates or harshness of old-style alkylating agents, but it brings irritant risk and potential environmental liability. Always use gloves and splash goggles, and keep transfers within a fume hood. Spilled powder creates fine dust that clings to benches or gloves, spreading residue wider than you might guess—wipe down surfaces and scan for strays after use. In case of accidental exposure, rinse skin thoroughly and seek further guidance from the MSDS. Safe disposal means collecting solid scraps for approved chemical waste handling, not ordinary trash or laboratory sink drains. Facilities often mandate secondary containment and accident logs, especially for intermediates used in regulated drug discovery.
Beneath the practical chemical traits, 1-Phenylsulfonylpyrrole makes a real impact in targeted synthetic campaigns. Medicinal chemists build it into scaffolds for kinase inhibitors, antifungal prototypes, and new antimicrobials, banking on its ability to survive rough reaction conditions. Electronic materials research calls on it for organic semiconductors, where protected core units demand stability during multi-layer device assembly. Academic labs value its role in multistep syntheses; once, during a project on fused heterocycles, I saw the sulfonylpyrrole carry a molecule through four separate transformations before finally being swapped out at the last step. Peptide science, too, has seen exploratory work, where pyrroles participate as building blocks or recognition units. The variety of patented pharmaceuticals and experimental OLED materials underlines the compound’s breadth.
Work on 1-Phenylsulfonylpyrrole marches forward in two main directions: fine-tuning the introduction/removal of the sulfonyl group and pushing application scope. Chemists now explore milder deprotection methods, hoping to shorten synthesis timelines and reduce byproducts. The compound’s electron-rich core draws in researchers in cross-coupling, polymer chemistry, and even bioconjugation. Recent papers highlight its role as a functional handle for radiolabeling, enabling pharmaceutical candidates to be traced in metabolic studies. The focus on greener synthesis—using less toxic reagents, recyclable solvents, and continuous flow—reflects both regulatory pressure and growing environmental awareness in the chemical industry. My experience in collaborative projects shows that the compound's versatility carries real weight: chemists who try it out often find new reactions or fields opening up unexpectedly.
Toxicological studies for 1-Phenylsulfonylpyrrole stem from its use in drug synthesis and production scale-up. Acute toxicity registers low under standard handling, with irritant effects showing up at higher exposure. In animal models, no alarming findings have appeared, but full profiles—chronic exposure, metabolic breakdown—still need more investigation, especially outside rodent systems. Environmental impact emerges more from production waste than use, since the sulfonyl group tends to persist longer in soil or water if mishandled. Regulatory agencies focus on workplace exposure, not widespread consumer risk, but new life-cycle analysis tools make this a moving target. From my work with EHS teams, one lesson sticks: vigilance with documentation and effluent management pays off, both for legal protection and community trust.
1-Phenylsulfonylpyrrole looks ready to hold a steady spot in synthetic strategy as chemistry leans further into complexity and efficiency. The search for more stable, versatile protecting groups for heterocycles is nowhere near finished, and this molecule finds itself at home in cycles of innovation: process chemists want more control, and materials scientists want new properties. High-throughput screening and automated reaction optimization now churn out derivatives and analogues, some heading into clinical trials or commercial electronics. Renewed attention to green chemistry challenges researchers to design easier removal methods for sulfonyl groups or to swap in less persistent analogues. As regulations tighten and the push for sustainability takes center stage, 1-Phenylsulfonylpyrrole will likely evolve—whether through new deprotection chemistry, improved synthesis, or by feeding innovation in the next wave of pharmaceuticals and smart materials.
1-Phenylsulfonylpyrrole isn’t something you find on a shelf in your kitchen or under your bathroom sink. In the world of chemistry, though, it’s a real workhorse, showing up in labs that focus on organic synthesis and medicinal research. Few people outside those circles talk about it, but those who do, know its value runs more than skin deep.
Researchers and students often focus on the big molecules when they talk about drug discovery, but much of the heavy lifting gets done by the smaller, modular compounds that help build those blockbuster molecules. I remember a postdoc explaining to me that chemistry is a lot like building with Lego blocks. 1-Phenylsulfonylpyrrole turns out to be one of those very handy blocks if you’re aiming to construct anything with a pyrrole core. That comes up more often than you’d think in cancer research and in creating molecules to target infections.
Digging into the facts, the pharmaceutical industry uses 1-Phenylsulfonylpyrrole as a building block for heterocyclic compounds, especially those needed in early drug development. Many drugs these days show off a pyrrole ring somewhere in their skeleton—think of anti-inflammatory prescriptions or even antivirals developed in the last decade. The phenylsulfonyl part makes this compound a favorite for triggering certain kinds of reactions. It helps chemists guide transformations more precisely, often boosting the safety and reliability of the process.
Getting more technical, 1-Phenylsulfonylpyrrole serves as a protecting group. During complex syntheses, chemists protect one part of a molecule from reacting while they change another section. This trick keeps reactions running smoothly and helps avoid expensive, time-wasting mistakes. The compound’s stability under a range of conditions also means it won’t disappear midway through a complicated, multi-step process.
Pharmaceuticals grab headlines, but this compound turns up in other spots too. Some agrochemical research teams look to it when crafting new pesticides or herbicides. The goal isn’t just to kill bugs and weeds: it’s about finding agents that break down safely in the environment and target specific threats without harming everything else. Pyrrole derivatives, made using 1-Phenylsulfonylpyrrole, have shown promise here. They let researchers test and tweak molecules until they find that sweet spot between effectiveness and safety.
I’ve talked to people working on materials science projects who mentioned using this same class of intermediates in crafting new dyes and specialty coatings. Organic electronics, those thin and flexible displays in smart devices or solar panels, sometimes rely on related chemistry. Even small tweaks in the molecular structure can mean the difference between a product that works and one that fizzles.
The usefulness of 1-Phenylsulfonylpyrrole comes with responsibility. Labs handle it with care, limiting exposure and following strict guidelines. That’s part of building trust—not only within the scientific community, but with people who depend on the benefits that come from this work. If we want to keep seeing new medicines, safer farm chemicals, and better electronics, we need research environments where safety gets as much focus as scientific excitement.
Looking ahead, scientists keep searching for new ways to use and improve these compounds. By sharing best practices and pushing for more sustainable methods, the research world helps turn a humble building block into something that supports health, technology, and daily life in ways most folks never stop to consider.
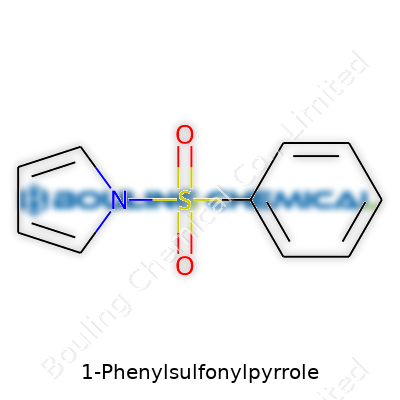
1-Phenylsulfonylpyrrole grabs my attention for its clever combining of two distinct worlds. On one hand, the molecule features a five-membered ring called pyrrole. Chemists often see pyrrole as the “workhorse” of nitrogen-containing rings. The structure boasts four carbons and a nitrogen, all tucked together; this arrangement fuels stability and hearty participation in a wide range of reactions. Add the phenylsulfonyl group at the 1-position, and the whole dynamic shifts.
Digging into the phenylsulfonyl part, we picture a benzene ring (six carbons linked in a hexagon, loaded with clean symmetry and resonance energy) attached to a sulfonyl group (a sulfur atom bound to two oxygens, with a double bond each, and another bond to an oxygen which ties it to the pyrrole). The sulfur-oxygen bonds pull electron density, which tugs at the electronics on the ring, changing the chemical behavior.
Through my research, this coupling of pyrrole and phenylsulfonyl isn’t just a textbook curiosity. Medicinal chemists often use sulfonyl groups when they want to increase water solubility or tweak the way molecules interact with biological targets. For instance, the addition of a sulfonyl group to a heterocyclic core like pyrrole usually helps with metabolic stability. It lowers the molecule’s basicity, steering interactions in the body’s slightly acidic pockets.
In my hands, working with sulfonyl derivatives has cut the time needed for purification. The bulk of the phenylsulfonyl group often bumps up the molecule’s melting point, making it easier to isolate and handle in the lab. Many colleagues turn to molecules like 1-Phenylsulfonylpyrrole as building blocks when creating new drug candidates or exploring chemical libraries.
Safety and environmental sustainability can’t go ignored. A sulfonyl group, being pretty robust, rarely breaks down into something reactive or toxic. I’ve noticed this characteristic fits in well with efforts to build safer chemicals that linger less in the environment. Institutions like the EPA keep a close watch on the breakdown products of complex chemicals. Phenylsulfonyl groups have a solid track record here, with fewer alarming byproducts compared to nitro or halo-substituted aromatics.
For anyone developing new materials or medicines, there’s always a balance: stability, performance, safety, and environmental friendliness. From my perspective, 1-Phenylsulfonylpyrrole ticks many boxes. Its structure—pyrrole with a robust phenylsulfonyl at the 1-position—offers solid performance in organic synthesis, day-to-day handling, and post-use environmental considerations.
Synthetic chemists looking to improve access to 1-Phenylsulfonylpyrrole keep refining routes, searching for fewer steps, cleaner reagents, and less energy waste. Catalysts that drive sulfonylation at just the right ring carbon, or direct coupling methods that avoid heavy metals, have become more commonplace. In my own experience, paying close attention to reaction pH and temperature can make or break a single step.
Younger scientists in training see molecules like this as the future—simple, functional, and responsible. The core lessons come down to thinking not only about what a molecule does but also how it fits our needs for safety, sustainability, and practical chemistry.
Every chemical on the shelf tells a story about risk and responsibility. 1-Phenylsulfonylpyrrole brings its own quirks. I'm not someone who ignores those warning labels or forgets the stubborn reality of what happens when a compound starts to degrade in the cabinet. I've seen what poor storage does—in labs, in industry, and sometimes in small, hopeful startups trying to edge out a discovery. So, let's dig into storing this compound in a way that avoids surprises, keeps people healthy, and protects investments.
Temperature matters more than many realize. Unpredictable heating in a room might push 1-Phenylsulfonylpyrrole past where it feels “comfortable.” Chemical and physical stability both hinge on predictable, low temperatures. Refrigerators do a good job at around 2–8°C. Folks who know their labs will go further, setting aside dedicated fridges just for organics like this. That habit isn’t a luxury, it’s a shield against product breakdown, changing color, or odor. Unexpected breakdown costs real money, delays research, and can ruin a day’s work in moments.
I learned early on to treat sunlight as an enemy to sensitive compounds. Sunlight doesn’t just glare through a window. It can build up heat inside a container and spark reactions nobody wants. Keeping chemicals in amber or opaque glass isn’t just tradition, it’s a trusted method passed from generation to generation. Moisture joins the list of threats. Shelf life drops off fast if a desiccator’s not part of the routine. Using silica gel packs inside a sealed container or placing materials in a desiccator preserves more than just stability—it protects your data, results, and peace of mind. Humidity has a way of sneaking in even if you’re careful.
No one benefits from mystery bottles. The worst chemical accidents in my experience came from a combination of carelessness and lost labels. Full identifiers, dates of receipt and opening, hazard notes; these details take moments to write, and they prevent all sorts of messes. Keep up with a logbook. Track how much you have, who used it last, and why. This discipline separates chaos from order and keeps research honest. Regulators check on this, too, and a tidy log avoids trouble if they do drop by.
Gloves, goggles, and lab coats aren’t there for decoration. Every time I’ve seen someone brush off these basics, I’ve watched them regret it later. Spills can happen with barely a nudge. Good containers matter as well. Instead of flimsy plastic, pick thick-walled glass with a tight-sealing cap. Don’t stack other bottles on top. Place the container on a stable, ventilated shelf away from acids, bases, or oxidizers. If an accident happens, easy access and organization can keep it manageable.
Waste is unavoidable in any lab. Never pour unused or spent 1-Phenylsulfonylpyrrole down a sink or let it sit forgotten. Use designated hazardous waste bins. Build a relationship with your university or facility’s hazardous waste team—they know the ropes and can save you from fines or environmental mistakes. Old stocks? Don’t hesitate—dispose of them responsibly instead of hoping for one last use. Caring for your lab’s chemical inventory — including safe storage and proper disposal—shows respect for colleagues, research, and the world outside.
No matter the research goal or the deadline, nothing trumps safety. 1-Phenylsulfonylpyrrole offers plenty of value in organic chemistry, especially in pharmaceutical and materials research. Yet, achieving results should never come at the risk of health or well-being. Practical experience around these chemicals shapes how I work much more than any textbook alone ever could.
Start with the basics—1-Phenylsulfonylpyrrole isn’t something to handle with bare hands or an open bench. Sulfonyl pyrroles, much like other aromatic compounds and sulfonyl derivatives, often irritate skin, eyes, and airways if released as dust or vapor. Some even provoke allergic reactions. Less obvious hazards creep in through chronic, low-level contact. I learned quickly in my student days not to underestimate lingering headaches or unexpected rashes. They’re often the first warning signs.
Gloves always come first. Nitrile serves well for most organics, and I avoid latex anywhere solvents or sulfonyls appear. Splash goggles keep eyes safe from accidents; regular glasses do not stop a chemical mist. Most important, lab coats and chemical-resistant aprons block spills that eat through fabric or skin. Folks tend to skip on shoe covers, but more than one ruined pair of sneakers convinced me those drips find feet fast.
Good airflow saves lab workers from most inhalation risks. Fume hoods make the biggest difference, especially for weighing, transferring, or heating the compound. Fans by the window or open doors never match up, and they put others at risk. Everyone who’s shared a cramped academic lab knows how heavy some vapors hang in the air—engineered draft still beats cracked windows every time.
I never overlooked storage, since so many issues start with the wrong kind of bottle on the wrong shelf. Dry, cool, and away from light—the old mantra for stability holds true here. Some sulfonyls react with acids or bases, so clear labeling and separation from incompatible chemicals stops surprises. Small containers cut spills and make every hazard easier to manage.
Disposal trips up even experienced teams. Pouring old chemicals down the sink never counted as an option where I worked. Dedicated disposal bins and coordination with hazardous waste services protect not just us but everyone downstream. I always checked the latest waste code requirements and kept records for peace of mind and inspection day sanity.
Every safe day builds from habits and shared knowledge. I learned most safety tricks from older colleagues generous with their stories of near misses and handy fixes for sudden splashes or faulty hoods. The best teams watch out for each other, sharing updated data sheets, flagging new hazards, and holding the line when shortcuts seem tempting. Regular refreshers, drills, and honest conversations kept our projects running and our people healthy.
Chemical safety always evolves. My own trust comes from seeing a culture that fixes problems early and never hides mistakes. It’s simple: each person’s commitment protects everyone’s hands, lungs, and futures. Handling 1-Phenylsulfonylpyrrole safely calls for more than checklists—it takes vigilance, open eyes, and a respect for the substance, every single day.
Anybody who spends time in a chemistry lab knows that purity matters a lot more than paperwork suggests. 1-Phenylsulfonylpyrrole, a valuable intermediate in pharmaceutical and organic synthesis, rarely arrives as a nameless white powder with ambiguous stats. The industry standard for this compound tends to sit at 98% purity or higher. Some suppliers reach up to 99% purity, especially when their target customers mix high-stakes research groups and global pharmaceutical companies.
A Certificate of Analysis (CoA) usually spells out how clean the batch really is; it doesn't only mention purity, but also lists water content, residual solvents, and trace metals. Imagine expecting 1-Phenylsulfonylpyrrole to stand up to a demanding synthesis, only to find impurities that shut down or detour an entire reaction. Contaminants—sometimes left over from manufacturing, sometimes creeping in from storage—can sabotage expensive projects. Laboratories focused on developing finished drug substances give a high rating to reliability and transparency from their suppliers, and rightly so.
Specs for this compound don’t only float around as numbers on a PDF. Chemists want to know melting points, solubility in common solvents, residual moisture, and particle appearance. Most labs report a melting point near 110°C. That check offers a fast first cut when confirming a purchase, especially if a deviation signals hidden contamination or degradation.
For solubility, this compound handles organic solvents such as DCM and ethyl acetate fairly well, which affects how easily it fits into downstream chemistry steps. Anyone planning to use 1-Phenylsulfonylpyrrole in a scale-up or pilot project would watch for solid specifications from the supplier. Sometimes, inconsistent particle shape or size doesn’t only frustrate logistics—it can jam up automated feeders and introduce variability on the production floor.
The big pharmaceutical buyers, the early-stage startups, and even university teams ask for batch-specific spectral data, including 1H NMR and LC-MS. With increasingly strict global regulations on nitrosamine and metal impurities, smart buyers look for suppliers including data on heavy metals (often less than 10 ppm lead, cadmium, or mercury) and solvent residues in their batch test results.
A solid supply chain avoids nasty surprises, especially for specialty chemicals. Problems sometimes stem not just from purity, but from poor specification controls. I’ve seen research projects stall for weeks because of off-spec byproducts or inconsistent crystalline forms. The solution, as I’ve learned through experience, often comes down to real conversations. Buyers reach out to suppliers, request extra analytical data, and sometimes insist on samples from multiple batches before placing a big order.
Quality management systems like ISO 9001 make a difference, but what really counts on the ground is accountability. The market features some suppliers with excellent technical support who welcome feedback and quickly address concerns—those are the ones repeat buyers stick with for years, even if the price is higher. A well-maintained documentation system lets a lab trace any issue back to the source, which lowers waste and builds trust.
Digital systems now help too. With blockchain or QR-coded tracking, some producers support real-time tracing of product lots, storing every test and movement for reference. This transparency helps make sure that each batch of 1-Phenylsulfonylpyrrole delivered to a lab doesn’t just claim purity, but demonstrates it, bottle to bench.
| Names | |
| Preferred IUPAC name | 1-(benzenesulfonyl)pyrrole |
| Other names |
1-(Phenylsulfonyl)pyrrole N-Phenylsulfonylpyrrole N-(Phenylsulfonyl)pyrrole |
| Pronunciation | /waɪˈfɛn.ɪl.sʌlˌfoʊ.nɪl.pɪˈroʊl/ |
| Identifiers | |
| CAS Number | [6367-35-7] |
| 3D model (JSmol) | `3Dmol.js?model=CC1=CC=CC=C1S(=O)(=O)N2C=CC=C2` |
| Beilstein Reference | 136215 |
| ChEBI | CHEBI:149364 |
| ChEMBL | CHEMBL185180 |
| ChemSpider | 19718459 |
| DrugBank | DB07401 |
| ECHA InfoCard | 100.108.948 |
| EC Number | 6367-34-6 |
| Gmelin Reference | 92697 |
| KEGG | C18755 |
| MeSH | D017964 |
| PubChem CID | 136892 |
| RTECS number | GE8400000 |
| UNII | CG6R8C91HF |
| UN number | 2811 |
| Properties | |
| Chemical formula | C10H9NO2S |
| Molar mass | 221.26 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.27 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 1.08 |
| Vapor pressure | 0.0000136 mmHg (25°C) |
| Acidity (pKa) | 5.65 |
| Basicity (pKb) | 13.43 |
| Magnetic susceptibility (χ) | -73.29×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.582 |
| Dipole moment | 4.71 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 385.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −86.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -5318 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07, GHS09 |
| Signal word | Warning |
| Hazard statements | H302 + H312 + H332 |
| Precautionary statements | P261, P264, P271, P273, P280, P302+P352, P305+P351+P338, P312, P332+P313, P337+P313, P362+P364 |
| NFPA 704 (fire diamond) | **1-1-0** |
| Flash point | > 152°C |
| LD50 (median dose) | LD50 (median dose): >2000 mg/kg (rat, oral) |
| NIOSH | GZ3150000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg/m³ |
| Related compounds | |
| Related compounds |
Pyrrole Phenylsulfonyl chloride Phenylsulfonylacetylene 1-Phenylpyrrole 1-Tosylpyrrole N-Methylpyrrole |