Back in the mid-20th century, discoveries around heterocyclic chemistry brought forward a new class of aromatic compounds that caught the interest of both academics and industry professionals. Among these, 1-Phenylpyrrole jumped out as a molecule with both versatility and potential. Its synthesis was driven by the search for better intermediates in pharmaceutical and agrochemical pipelines. Chemists sought structures that could bridge functionality and stability. With the aromatic pyrrole core and attached phenyl ring, this compound found its way into hundreds of laboratory notebooks, each test hinting at applications from material science to medicinal chemistry. Years of research, small breakthroughs, and plenty of trial and error placed 1-Phenylpyrrole into the repertoire of standard lab compounds by the 1970s.
In the lab or in the warehouse, 1-Phenylpyrrole most often appears as an off-white to faintly yellow crystalline powder. Its formula, C10H9N, tells a story of simplicity but hides plenty of possibilities in terms of reactivity and modification. It sits comfortably between the worlds of intermediate and building block. Whether working on new light-emitting materials, tweaking pharmaceuticals, or exploring possibilities for advanced polymers, it offers value in the chain of development. Anyone working with aromatic amines or heterocycles has likely entered its CAS number – 1003-29-8 – into their notebooks more than a few times.
1-Phenylpyrrole melts at around 54-56°C and boils close to 300°C under atmospheric conditions. Its density hovers near 1.1 g/cm³, giving it a substantial, manageable feel during transfers and preparations. Its solubility leans toward organic solvents and keeps clear of water. I’ve seen it dissolve swiftly in ethanol and acetone, requiring little encouragement with gentle warming. The molecule brings together stability and slight reactivity, holding up under normal storage conditions, but its nitrogen atom still offers anchor points for electrophilic substitution or complexation.
Sourcing 1-Phenylpyrrole from reputable vendors, you look for purity specs around 98% or higher. Technical data sheets break down typical residue levels, moisture content, and trace impurities – mostly to reassure users who need strict control for pharmaceuticals or photonics. Standard bottles feature clear hazard labeling: avoid ingestion, inhalation, and prolonged skin contact. For those in regulated industries, the documentation usually checks out with details from titer assays and spectroscopic analysis.
Most standard preparations begin with pyrrole and bromobenzene through a palladium-catalyzed cross-coupling, often a Suzuki-Miyaura or Buchwald-Hartwig reaction in today’s workflows. The process calls for dried solvents, inert atmosphere, and careful exclusion of water. Yields often climb above 80% if the catalysts are fresh and reaction parameters tuned. Purifying the crude product involves routine chromatography, crystallization, or sometimes distillation, depending on scale and downstream requirements. Once isolated, the compound can be stored in amber glass with a tight seal to keep out moisture.
1-Phenylpyrrole stands as both a participant and product in many synthetic routes. Its structure handles substitution at the pyrrole ring with a range of electrophiles. Adding electron-withdrawing groups to either ring changes the compound’s optical and redox properties, so chemists tinker with nitro, halogen, or amino substitutions when targeting custom dyes or sensors. The nitrogen atom supports alkylation, sulfonation, and acylation, broadening the field. For those invested in organic electronics, modifying the phenyl group opens possibilities for π-conjugated systems and new organic semiconductors.
Searching catalogs, scientists might find 1-Phenylpyrrole under several labels: Phenylpyrrole, N-Phenylpyrrole, or CAS 1003-29-8. It turns up as a reference compound for pyrrole derivatives, and, in some circles, as NSC 146428. While shopping globally, language translations occasionally add spelling twists, but the compound always circles back to the same IUPAC core.
Working with 1-Phenylpyrrole brings common laboratory precautions into play. I’ve worn gloves and used dedicated glassware to avoid unnecessary exposure. The material doesn’t match the volatility of some amines, but its dust may cause irritation to skin, eyes, and respiratory tract. Labs install exhausts or use gloveboxes during synthesis to cut risk. For waste, incineration in a controlled facility fits the best practice, making sure trace organics don’t end up in water supplies. Anyone training new chemists should emphasize correct labeling and routine MSDS review, not just for compliance but for safety.
The reach of 1-Phenylpyrrole stretches through academic research, pharmaceuticals, electronics, and specialty materials. In drug discovery, it pops up as a scaffold in structure-activity-relationship studies, where tweaks to the core molecule can lead to antiviral or antimicrobial candidates. The material science world, always hungry for new building blocks, uses its aromaticity for photoluminescent compounds, OLEDs, and molecular sensors that respond to light or electricity. Certain insecticides and fungicides have seen improved efficacy by including pyrrole derivatives, giving the agriculture sector new options for resistance management. I’ve spoken with polymer chemists who swear by N-substituted derivatives for fine-tuning conductivity in experimental batteries. Each field finds a way to sharpen or repurpose its functionality, rarely letting a kilogram go to waste.
Across university labs and R&D divisions, 1-Phenylpyrrole acts as a springboard for building complexity. Its ring system forms the basis for combinatorial libraries, while the aromatic groups draw attention for π-stacking interactions needed in supramolecular chemistry. The rise of machine learning in molecular design also features this compound as a training data point, helping guide algorithms to predict photophysical behaviors or binding affinities of novel derivatives. My own experience as a research assistant brought me into contact with automated reactors exploring a dozen new pyrrole analogs each month, all cataloged down to the NMR peak and UV-vis response. Such digital-driven approaches have expanded what used to take weeks into work that now wraps up in a long afternoon.
Toxicologists approach 1-Phenylpyrrole with measured caution. Rodent studies show fairly low acute toxicity, but concerns remain about chronic exposure in industrial settings. Some in vitro assays hint at moderate cytotoxicity when concentrations climb high, particularly on liver cell cultures. As the molecule undergoes metabolic breakdown, a few oxidative products can interact with DNA or proteins, calling for continued surveillance in pharmaceuticals and environmental releases. Most safety screens set acceptable daily intakes much higher than typical worker exposure, but ongoing assessments track metabolic byproducts, mutagenicity, and potential endocrine interference. My impression after consulting a few safety data sheets and case studies: it pays to use common sense, ventilation, and protective gear.
Upcoming years promise fresh discoveries as attention shifts to next-generation electronics and sustainable chemistry. Demand for efficient, customizable light-emitting materials should grow, with 1-Phenylpyrrole analogs appearing in more commercial OLED panels and smart sensors. Copper or iron-catalyzed versions of classic cross-coupling reactions could trim synthetic costs, making custom derivatives affordable for energy storage and medical imaging. Environmental scientists push for greener production, with enzymes or recyclable catalysts entering industrial protocols. Meanwhile, research into biological activity continues, seeking derivatives that balance efficacy with safety across medicine and crop protection. If one thing stands out, it’s how the simple pairing of a phenyl and a pyrrole keeps spurring creative work decades after its discovery. As regulations shift and priorities change, this molecule promises to keep its place on chemists’ shelves, a reminder that even small, unassuming structures may unlock future technologies.
1-Phenylpyrrole doesn’t often show up in everyday conversation unless chemistry class is calling. Yet, its structure and behavior reveal plenty about how organic molecules are pieced together and why small tweaks in design change everything about how a compound acts.
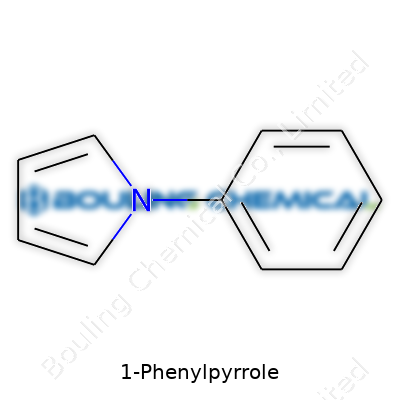
This molecule stands out for a couple of reasons. Picture a five-membered ring: that's pyrrole, where four carbons round out the shape, and a nitrogen closes the loop. Attach a benzene ring right onto that pyrrole, specifically at the nitrogen’s neighbor spot, and there you have it. The chemical formula goes as C10H9N. Its framework ties together two familiar faces from organic chemistry: pyrrole and benzene. It’s like chemistry’s version of a crossover episode.
The structure of 1-Phenylpyrrole isn’t just some academic exercise. Chemists in research labs build on top of scaffolds like this one for lots of reasons: drug research, organic electronics, and sensing applications. The twist and angle between the two rings can steer how well electrons zip around the molecule or where it fits with others. Sometimes, a simple bond sets up a whole path for discovery.
I remember handling 1-Phenylpyrrole as a grad student, our bench cluttered with glassware and yellow post-its. The compound itself seemed unremarkable, just powder at the bottom of a vial. Getting a good look under a crystal structure analyzer—now that was something else. The benzene ring almost floats beside the pyrrole, like it’s undecided on how close it wants to get. That separation matters. We found that charging up the system or shining UV light shifted everything, hinting at uses in sensors or light-based tech.
This chemical isn’t stuck in textbooks. Its geometry acts as both barrier and bridge, controlling how molecules talk. Researchers tracking environmental pollutants use it as a model for how certain substances spread through water and soil. Switch up a bond angle, tweak a substituent, and suddenly the molecule absorbs light different, sticks to surfaces, or dances with proteins in unexpected ways. Lessons from 1-Phenylpyrrole show up in new dyes, novel drug candidates, and tools for tracking chemical pollution.
The real opportunity starts once chemists get out of rote memorization and begin pushing molecules like 1-Phenylpyrrole into unexpected territory. Careful mapping of its atomic arrangement helps predict which tweaks might unlock new reactions or bring cost-effective materials to electronics and environmental testing. Open-access chemical databases and molecular modeling have made it easier than ever to simulate and predict how alterations in the structure could shift performance.
For those hoping to engineer smarter materials or design greener chemicals, it comes back to learning how molecules like this one put their atoms together. It’s not just about drawing hexagons and pentagons on paper — it’s about questioning how these shapes affect what we can do in medicine, technology, and sustainability. More research funding, better collaboration between chemists and engineers, and a focus on connecting structure with function will get more out of molecules like 1-Phenylpyrrole.
Ask most people about 1-Phenylpyrrole and you’ll probably get a blank stare. Out in the real world, though, this chemical compound finds its way into places we use every day, even if the label never tells us so. In my own days flipping through research from my chemistry minor, I spotted 1-Phenylpyrrole popping up across a spread of research journals and industrial reports, suggesting that even low-profile molecules can undergird surprising things.
Countless modern gadgets, from laptops to lighting, rely on materials that know how to move electrons with some style. 1-Phenylpyrrole brings a stable backbone for the field of organic electronics. Builders of organic light-emitting diodes (OLEDs) look for molecules that create strong, reliable charge transport; 1-Phenylpyrrole fits right in. Some OLED screens, those thin, bright displays that set new standards for smartphones and televisions, depend on similar compounds. The same goes for some solar cell materials, especially newer ones that blend organic chemistry with cheap manufacturing. Anything that helps lower costs or boost the life span of an OLED display stands out in a market where every year feels like a leap forward.
Synthetic dyes and pigments make up another area where 1-Phenylpyrrole quietly shows its value. Color designers need building blocks that offer strength and tunability. On factory floors producing textiles or plastics, chemists go for structures that resist fading and deliver vibrancy, especially when the sun or washing machine gets involved. 1-Phenylpyrrole serves as a starting point for a range of colorants. Take a stroll down any city street and you'll see shopfronts and fabrics that rely on such foundational chemistry.
Medicinal chemists regard heterocyclic compounds—the family that includes 1-Phenylpyrrole—as promising scaffolds for drug design. Structurally, pyrroles pop up in quite a few natural and synthetic biologically active molecules. Labs working on anti-inflammatory agents, anti-tumor prospects, and more have run explorations starting with 1-Phenylpyrrole derivatives. My workbench in grad school never touched this specific molecule, but I clearly recall the energy whenever a new pyrrole-based structure showed positive results in cell tests. While drug discovery remains a maze, any structure that broadens the arsenal gets real attention.
For folks with a love for visual science, 1-Phenylpyrrole’s other quirk is its use as a probe in research. Its sensitivity to its environment—basically, how its properties change in different solvents—makes it useful for understanding molecular interactions or the subtle dance of electrons in more complex systems. Labs probing molecular environments or studying basic electron transfer processes keep returning to this molecule for its responsiveness. Knowing which molecules play nice with water or other solvents turns out to matter a lot, especially as researchers look for cleaner, greener solvents in manufacturing.
Industry loves a backbone that’s both stable and flexible. The lesson here? A molecule built on a simple ring and an attached phenyl group provides options. If chemists can tweak this structure further, new applications could open up—maybe a safer colorant, or a more efficient path toward printable electronics. It pays to keep an eye on the overlooked. Sometimes, big impact comes from small places.
1-Phenylpyrrole isn’t something you find on the corner shelf. As an organic compound, it has a mix of uses in research and industry. Its molecular structure means it can react with air, light, and moisture in subtle ways. Having worked in a small lab that kept a steady supply of aromatic compounds, the unpredictable nature of substances like this taught me respect early on. Even traces of moisture or sunlight can shift how the chemical behaves, bringing risks nobody wants.
Chemicals don't take care of themselves. For 1-Phenylpyrrole, glass containers with tight, chemical-resistant lids keep things where they should be. Polyethylene and polypropylene often leach or deform, so glass jars work better. Dark bottles reduce light exposure — this isn’t just lab perfectionism; light makes many nitrogen-based organics degrade or sometimes make byproducts you don’t want to breathe.
I’ve seen people stash a bottle of this compound in a refrigerator alongside lunch — not a good move. The right storage temperature sits below room temperature, but household fridges collect moisture. Dedicated chemical fridges, set dry and kept organized, help keep this compound stable. Not much smells worse than oxidized pyrroles if you slip up.
Polymer gloves and safety specs serve as the front line, but not the whole defense. Wearing a proper lab coat, working in a hood with ventilation, and having a spill kit ready all matter. I remember a story from a peer who didn’t notice a drop slipped on the outside of his glove. It took minutes before he realized, and that brief contact triggered dermatitis.
Planning every action gives a big payoff. Lay out your tools, double-check labels, work slowly. Fume hoods matter a lot here. Vapors can build up, even from ‘closed’ containers, so turning on the airflow isn’t just about comfort — it cuts the risk of exposure or fire.
1-Phenylpyrrole can catch fire more easily than many realize. Keep ignition sources away: this means no open flame, no hot plate without spark protection, no cell phones on the benchtop. I’ve met researchers burned by assuming the risk was small because the bottle looked sealed. Standard sand buckets and extinguishers give real backup if things go sideways.
Spill control plans don’t seem exciting until you actually need one. Small quantities of 1-Phenylpyrrole can make a mess if they escape containment. Cleanup works best with mineral absorbents, not paper towels. Once, during a small spill, clay absorbent saved a day’s work — and shoes — from permanent staining.
Without experience or close attention, someone might miss the less obvious hazards. Even seasoned researchers trip up. Training new staff every few months, running drills, and swapping old labels matter more than fancy alarms or expensive inventory software. Every safe, experienced team outperforms a fancy but careless setup.
Regulations give a framework, but safe habits and a culture of care give lifelines. Everyone, from students to seasoned chemists, stands to benefit from ongoing reminders and good systems. Wrong storage or lazy handling cuts into safety, experiments, and health far too often.
If you try to order 1-Phenylpyrrole, the first detail the supplier waves under your nose is the percentage next to the product name. That number is not fluff. Purity sets the mood for everything downstream—how researchers handle their experiments, how companies process material, and whether a project hits a snag. From the chemistry community to electronics to dye manufacturers, people dive into the catalog expecting to see high numbers. The standard ranges sit at 95%, 98%, and 99%. It’s easy to overlook those digits and see them as almost interchangeable, but anyone who’s ever fought with an impurity peak on an HPLC readout knows those stray molecules ruin reproducibility and add hassle.
You find 95% in places that want to save on budget but don’t get tripped up by a little extra weight on the contaminant side. If you’re screening molecules and only testing basic behavior, you might live with 95% because trace contaminants often don’t derail the story. For people like me, who have tried to run synthesis with low-purity starting points, the aftermath isn’t pretty. You end up guessing at color changes, suspecting secondary reactions, or chasing mystery peaks in your spectra.
The uptick to 98% might sound modest, but those two little percentage points slice headaches in half. In research, 98% has become the go-to for most synthetic work because reactions behave the way the book says they should. Many of us have stood by the chromatograph, breathing easier when the peaks come clean, rather than seeing a ghost signal from some leftover additive.
Once purity crosses the 99% mark, you’re living in a world of precision and problem-solving. Electronics use 1-Phenylpyrrole at this level because semi-conductors and OLEDs tolerate nothing less. Anyone prepping a material for light-based tech wants to avoid the scattered interference from background junk, which creeps in even if it’s just a fraction of a percent. Some labs push for 99.5% or above. It costs extra, takes more work from the supplier, and brings a batch size down, but these batches land in critical applications.
If you’ve ever seen data wrecked by a 0.5% contaminant, you stop questioning those premium price tags. For pharma, even higher grades might surface—often called “analytical” or “ultra-pure”—even though this pushes costs further.
Suppliers don’t just wave a magic wand over a bucket of powder. Achieving pure product means extra purification rounds, tighter control over process solvents, and more vigilant packaging to guard against environmental sneak-ins like dust or vapor. Distillation, recrystallization, and chromatography all play a part. It takes real-world effort and savvy, not just machinery.
I’ve had batches arrive at my workbench with tiny labels stating “98%,” and one with “99%.” Testing each for downstream use, the difference wasn’t theoretical. The 99% powder made reactions hum. Fewer re-dos, lower unpredictability, less troubleshooting. Less frustration that my hard work would be undone by something I didn’t cause.
If you want reliable results, ask plenty of questions before buying. Don’t let the catalog fool you into thinking all “98%” claims stack up evenly. Request certificates of analysis. Push for information on assay method—GC, HPLC, or melting point—because some impurities hide from certain tests. If purity matters for your goal, don’t just look at the number; look for transparency.
Demand for 1-Phenylpyrrole runs across a sliding scale, and so should the approach to purity. Chasing better quality sometimes stretches the budget, but in this field, every percent can mean the difference between frustration and breakthrough.
People in materials science or specialty manufacturing hear of 1-Phenylpyrrole and, right away, they start thinking about the hurdles of sourcing. This compound, known mainly as a photoinitiator and an electronic intermediate, isn’t something you’ll find sitting on warehouse shelves in every industrial park. Having spent some time tracking down obscure chemicals for research in the past, I’ve seen companies run into major headaches over procurement. It always boils down to two key factors: cost and reliability.
On paper, chemical suppliers list 1-Phenylpyrrole for sale, but seeing it on a catalog doesn’t always translate to true “bulk” access. Many suppliers, especially those in Europe and North America, shy away from keeping large stocks unless orders justify the investment. You’ll find bottles and small drums meant for research and pilot runs, sometimes up to the kilo scale, priced for the laboratory and not the production floor.
Larger batches, say fifty kgs and above, bring a different story. Major suppliers like Sigma-Aldrich or TCI will fill out quotation forms and ask about end uses, shipping destinations, and whether your facility is properly certified. Some chemical distributors in China and India step up with more flexible batch sizes, often supporting custom synthesis for bigger requests. It’s worth noting that dealing with offshore suppliers often means wrangling with language barriers, questions about purity, and uncertainty about delivery timelines—issues I’ve faced myself when ordering outside my home country.
Anyone who’s seen a purchase order balloon once you add on international shipping, tariffs, and hazardous material handling fees can confirm: the listed price tag for 1-Phenylpyrrole barely scratches the surface. The real wallet-buster comes in compliance checks, especially for companies governed by strict environmental or workplace regulations. I’ve seen teams chase their tails trying to update safety data sheets, train workers, or build safe storage for new hazardous goods just to bring in one niche molecule.
There’s also the headache of sample-to-scale variance; a compound that behaves perfectly in a five-gram test run may react differently at multi-kilo amounts, and this doesn’t always reveal itself until the shipment has cleared customs. I remember dozens of conversations with process engineers, frustrated that a promising synthesis ground to a halt over impurities or inconsistent lot quality.
Industry groups urge more transparency from suppliers. Every facility ordering semi-bulk or bulk quantities could benefit if more vendors were straightforward about real stock levels and lead times. A central marketplace for specialty materials, showing batch sizes, certifications, and estimated delivery windows, would save a lot of back-and-forth.
Pooling procurement through consortiums also makes sense. Smaller manufacturers could link up, put together joint purchase orders, and negotiate better terms—not just price, but quality guarantees and after-sale support. During my time sourcing solvents for an academic lab, we managed to piggyback on a university system’s buying group to land fair rates and better documentation.
Looking ahead, scaling the local production of key intermediates like 1-Phenylpyrrole could lower risk and improve pricing. Contract manufacturing partners based closer to home might not always match the lowest offshore quote, but the value of clear communication and reliability on recurring orders usually outweighs penny-pinching.
There’s no silver bullet here; bulk chemicals like 1-Phenylpyrrole follow the ups and downs of wider supply chains. Anyone entering this market, especially startups or research-driven companies, should budget extra time and elbow room in their production timelines. Those who get creative with sourcing strategies—and keep the lines open with trusted suppliers—find themselves in a much better spot when the next big project comes calling.
| Names | |
| Preferred IUPAC name | 1-phenyl-1H-pyrrole |
| Other names |
1-Phenyl-1H-pyrrole N-Phenylpyrrole |
| Pronunciation | /ˈwʌnˌfiːnəlˌpaɪˌroʊl/ |
| Identifiers | |
| CAS Number | 1003-84-9 |
| Beilstein Reference | 120924 |
| ChEBI | CHEBI:52055 |
| ChEMBL | CHEMBL127474 |
| ChemSpider | 203011 |
| DrugBank | DB08499 |
| ECHA InfoCard | 100.012.867 |
| EC Number | EC 221-979-7 |
| Gmelin Reference | Gmelin 84355 |
| KEGG | C16214 |
| MeSH | D000926 |
| PubChem CID | 70654 |
| RTECS number | UK5445000 |
| UNII | 59FQ1728FJ |
| UN number | UN3077 |
| Properties | |
| Chemical formula | C10H9N |
| Molar mass | 169.22 g/mol |
| Appearance | Pale yellow to brown solid |
| Odor | Aromatic |
| Density | 1.101 g/cm³ |
| Solubility in water | slightly soluble |
| log P | 1.92 |
| Vapor pressure | 0.0515 mmHg (25 °C) |
| Acidity (pKa) | pKa = 0.8 |
| Basicity (pKb) | 2.81 |
| Magnetic susceptibility (χ) | -56.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.612 |
| Viscosity | 1.17 cP (20°C) |
| Dipole moment | 2.30 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 365.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | 87.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4245.8 kJ·mol⁻¹ |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P273, P280, P302+P352, P305+P351+P338, P312, P321, P332+P313, P362+P364, P501 |
| Flash point | 86°C |
| Autoignition temperature | 570°C |
| Explosive limits | Lower: 1.4% Upper: 7.2% |
| Lethal dose or concentration | LD50 (oral, rat): 480 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 >2000 mg/kg |
| NIOSH | NIOSH: DJ9272000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | Boric acid, Buffer, Sodium carbonate |
| Related compounds | |
| Related compounds |
N-Phenylpyrrole 2-Phenylpyrrole Pyrrole Diphenylpyrrole Phenylpyrrolidine |