Long before 1-N-Boc-Piperazine appeared in today’s chemical catalogs, piperazine itself had made the rounds in both industrial and pharmaceutical circles. Chemists have recognized the unique, stable backbone of piperazine for decades, often as a jumping off point to developing active pharmaceutical ingredients and new materials. The move to protect the nitrogen with a tert-butyloxycarbonyl (Boc) group gave labs something new—better control over reactivity, easier handling, and hassle-free purification steps. In the 1970s and 80s, Boc protection gained traction, especially as medicinal chemists tried to create and test hundreds of analogs for drug development. People with hands-on lab experience know Boc-piperazine survives many conditions that leave other amine-protecting groups struggling, and that simple acid work-up has made deprotection convenient even during marathon synthetic campaigns.
1-N-Boc-Piperazine brings reliability to the table. It shows up as a white to off-white solid, so there’s no guesswork about purity in the bottle—chemists can tell right away if they’ve achieved a clean product when they’re running reactions on the bench. Labs store it in air-tight containers, and it tolerates brief air exposure without drama. It’s much favored for building drug molecules, serving as a protected building block for stepwise synthetic strategies. For any bench chemist, it’s a relief to skip chromatographic purifications thanks to Boc’s distinctive chemical resilience and ease of removal later.
This compound, C9H18N2O2, tips the scales at 186.26 g/mol. It’s not volatile, and doesn’t give off strong odors, which is always a plus on a crowded bench. It melts comfortably around 90–95°C. It will dissolve in common organic solvents like dichloromethane and ethyl acetate, and if forced, some chemists coax it into DMF or DMSO for tougher coupling reactions. Its stable carbamate group keeps it from reacting at the drop of a hat, so it won’t accidentally sabotage neighboring reactions or degrade in storage.
Labs order 1-N-Boc-Piperazine and track specs like purity (typically over 97% by HPLC or NMR), melting point, presence or absence of residual solvents, and water content by Karl Fischer analysis. Vendors stick to standardized shipping labels, often including GHS hazard warnings even when risks are minimal. It rarely carries hazardous transport codes, making supply chain logistics less complicated compared to some of its reactive cousins. A sharp researcher always checks the Certificate of Analysis (CoA) prior to running a new batch, knowing small discrepancies can derail milligram-to-gram scale synthesis downstream.
Chemists typically prepare 1-N-Boc-Piperazine by reacting piperazine with di-tert-butyl dicarbonate (Boc2O) under mild base—usually triethylamine or sodium carbonate in aprotic solvents. With a little cooling, Boc2O stably caps one of the two secondary nitrogens, while the other remains open for further transformations or couplings. Even undergraduate organic labs cover similar chemistry, since it illustrates crisp selectivity on symmetric molecules. Experienced practitioners watch for overreaction—double Boc protection lurks if too much Boc2O is present or reaction times run long.
Chemists rely on the Boc group for its cooperative behavior. You can strip it off cleanly with trifluoroacetic acid or hydrochloric acid in organic solvents, so any synthesized intermediate can easily reveal that free piperazine nitrogen for alkylation, acylation, or drug-like extension. Researchers in pharma leverage this to introduce radiolabels, fluorescent tags, or pharmacophores late in the synthesis. For anyone working without million-dollar purification systems, the Boc strategy saves both time and headaches, offering crisp separations with minimal side products.
If you’re scouring catalogs or literature, 1-N-Boc-Piperazine comes under several guises: tert-butyl 4-piperazinecarboxylate, N-Boc-piperazine, Boc-piperazine, or 4-(tert-butoxycarbonyl)piperazine. Each name points back to the same reliable molecule. Commercial sources offer various pack sizes as this building block finds widespread use in both academia and industry.
Experienced chemists respect Boc reagents but don’t handle them with undue fear. Use gloves and standard PPE—contact with skin and eyes isn’t catastrophic, but routine safety habits save trouble. Inhalation risk is almost nil, owing to its low volatility. Still, spill cleanups can be a nuisance on the bench. Waste management follows standard protocols for relatively innocuous organics. Large-scale facilities keep an eye on dust formation or unwanted decomposition products, but those risks rarely cross the mind of bench-scale workers.
The major pull of 1-N-Boc-Piperazine centers on medicinal chemistry. This molecule finds itself in the early stages of making new central nervous system drugs, kinase inhibitors, and all sorts of probe molecules for molecular biology. Biotech startups keep stocks on hand for rapid analog generation, since speed in synthesis can mean the difference between breakthrough and burnout. Beyond pharma, it crops up in agricultural screening, advanced materials research, and peptide science. The single protected pole allows modular construction of branched compounds or macrocycles—a major asset for researchers aiming to build complexity in single pots.
In the world of pharmaceutical R&D, 1-N-Boc-Piperazine enables what’s often called parallel synthesis. Chemists assemble large libraries of molecules, each bearing minor tweaks, and Boc-piperazine helps maintain fidelity across these variants. In fragment-based drug discovery approaches, the Boc group acts like a bookmark, allowing for derivatization or amplification only after preliminary screening narrows the field. Lab teams praise its adaptability and predictability—traits prized in research settings where reproducibility drives publishing, patenting, and, ultimately, regulatory approval.
On the toxicity front, 1-N-Boc-Piperazine sits well behind nastier reagents. It avoids the hazardous category in most official lists, though concentrated solutions can still irritate skin or mucous membranes with sloppy handling. Chronic exposure studies remain limited, given the compound doesn’t find use in consumer products or production-scale chemistry. Most labs emphasize mitigation by simple best practices—fresh gloves, dedicated glassware, and routine disposal of residues. As with many protected amines, long-term inhalation or ungloved exposure is discouraged, but formal tox data from animal models remains sparse compared to finished pharmaceuticals.
Chemists and process engineers continue to look for greener and more efficient ways to prepare, use, and recycle protected amines like 1-N-Boc-Piperazine. Regulatory scrutiny grows on solvents and waste streams, so the shift toward eco-friendlier reagents and solvents could shape next-generation Boc chemistry. Automated synthesis machines depend on reliable, shelf-stable building blocks—and 1-N-Boc-Piperazine fits this emerging workflow perfectly. As artificial intelligence and machine-driven discovery start playing bigger roles in drug invention, this molecule’s predictable behavior and easy handling will put it in an even brighter spotlight.
High purity chemicals don’t just spark pride in lab workers—they also shape research outcomes and manufacturing success. Anyone who tries to run a reaction with off-brand or questionable material knows the feeling. A reaction that runs smooth one month suddenly gives you gunky byproducts the next. The difference often boils down to what’s actually in the bag besides the name on the label.
For anyone handling 1-N-Boc-Piperazine, purity usually means how much of the white, crystalline solid matches up to the chemical formula C9H18N2O2, and how much strays from it. Commercial suppliers typically sell this protected piperazine derivative at over 98% purity, and some samples run above 99%. Anyone working in pharma, materials chemistry, or peptide synthesis knows why this matters. Even one percent off introduces enough impurities to clog up a purification column or to make analytical results go sideways.
I’ve seen stocks contaminated by leftover reagents, unclever handling, or even just moisture in the air. For 1-N-Boc-Piperazine, common hitchhikers include free piperazine, Boc anhydride residues, or bits of unreacted starting material. Sometimes users spot faint discoloration or catch a whiff that doesn’t match the bland, sorta sweet norm. One time in the lab, a supplier batch arrived sprinkled with what looked like baking soda. Tried to run a coupling with that, and the entire vessel fizzed out, wasting an afternoon.
It’s easy to balk at premium prices for 99%-plus grades. Plenty of folks look for bargains, tempted by lower-purity stocks. But just a little contaminant in 1-N-Boc-Piperazine, especially in small molecule synthesis, often causes purification nightmares. Extra chromatography or re-crystallization piles up work, slowing down projects and slashing yields. Some might think purification can fix everything, but materials full of related impurities or analogues won’t always cooperate, and the waste can sting tight budgets.
Most chemical suppliers ship their material with a certificate of analysis, listing factors like melting point, HPLC purity, and possibly NMR data. I always check those numbers before trusting an order. It’s tough to eyeball quality—a powder can look legitimate but still fail standards. Running thin-layer chromatography or a test NMR on a fresh batch became routine for my old group. A single unknown spot or unexpected peak often points to more trouble down the line.
Dealing with low-quality chemicals wastes more time than almost anything else in research. I’ve learned to stick with reputable suppliers and to store reagents in a cool, dry place—especially ones like 1-N-Boc-Piperazine, which don’t like getting damp. In-house purification sometimes helps, but for many, buying verified, high-purity stocks avoids all sorts of troubleshooting.
Across drug discovery and materials science, the purity of your chemical building blocks can shape safety, repeatability, and downstream progress. Chasing the best grade lifts the reliability of every experiment. Besides, nothing feels worse than finding out after a month of work that a low-grade batch spoiled the original plan from the start.
There are tons of chemical compounds out there, and each one comes with its own quirks. 1-N-Boc-Piperazine isn’t fresh out of a sci-fi novel, but for synthetic chemists, it ranks as a staple. Popular in pharmaceutical labs and everywhere someone handles medicinal chemistry, it does a simple thing: help build other molecules. Keeping it in top shape calls for a bit of hands-on care—more than just sticking the bottle on a random shelf.
If you read “store at room temperature” and think that means any old temperature will do, you’re rolling the dice. I’ve seen plenty of experiments fail just because someone’s “room temperature” crept past 25°C in a hot lab. 1-N-Boc-Piperazine tolerates standard lab conditions, so keeping it between 15–25°C keeps things steady. Some folks get nervous and toss every chemical in the fridge, yet moisture and condensation are no friends to this compound. Unless a supplier’s label screams for cold storage, a dedicated chemical cabinet will do just fine—keep the bottle out of direct sunlight, away from heat sources, and you’re on the right track.
I learned early on that loose caps can ruin more than a day’s work. Air brings more trouble than most realize, sneaking in little bits of water vapor and contaminants. 1-N-Boc-Piperazine, like many organic compounds, fares better in a dry, closed container. Don’t assume the cap is tight just because it looks snug. Give it the twist test. Some labs use glass bottles with rubber-lined caps for an extra layer of defense, especially in damp environments. Never store it next to strong acids or bases—the vapors can drift over and trigger slow reactions that spoil purity.
Even if you can’t spot a puddle, the air carries more water than you think. Scoop out portions quickly, close the lid, and store desiccants nearby if the weather gets muggy. Silica gel packets aren’t just for fancy shoes—they keep powdery materials dry and clump-free for longer stretches. One summer in a poorly ventilated stockroom taught me the hard way: with too much moisture, organic compounds can cake, discolor, or even start to degrade.
Many accidents come not from bad chemicals, but from simple mix-ups. Applying fresh labels with open dates, purity, and hazard notes give you a fighting chance against accidental misuse. Legible labeling saves hours and in some cases, prevents hazardous surprises. After multiple years in shared laboratories, I’ve stopped trusting faded or handwritten pencil marks for anything I do regularly.
For those who only use small batches, avoid ordering bulk bottles. Smaller containers keep product fresh and lower the risk if something spills. Stick to cabinets dedicated to organics, far from oxidizers or flammable solvents that might turn a bad day into an emergency. Ventilated cupboards or those with fire suppression are better than shoving containers under a sink for convenience’s sake.
Problems crop up most often from forgetfulness or overcrowding. One fix: post clear signage in storage rooms about best-practices for humid days. Rotate stock so that old materials don’t outlast their shelf life. Updating inventories every season helps prevent expired or contaminated compounds from lingering. Investing in better cabinets and environment sensors pays off by reducing costly lab downtime from ruined supplies.
Keep storage simple: cool, dry, away from light and reactive neighbors. Seal the lid, label the jar, and check stock often. Good practices here mean less waste and fewer headaches for everyone working in a shared space.
People outside research and manufacturing circles rarely talk about CAS numbers, but those numbers mean as much to chemists as car VINs mean to mechanics. Every compound gets a unique badge, letting anyone identify its molecular structure anywhere in the world. For 1-N-Boc-Piperazine, that badge is 57260-72-7. This doesn’t just help chemists find compounds in a database. It cuts down on mistakes, saves time, and sometimes prevents accidents in a lab.
Plenty of folks, when handed a bottle or a supplier’s product list, just want to know if what’s inside matches the order. Labels like 1-N-Boc-Piperazine seem straightforward, but chemicals have a habit of hiding behind similar names. Piperazine derivatives all look alike at a glance. The CAS number 57260-72-7 wipes out that confusion. Plug it into a database, and you get a single hit pointing to the right compound.
Back in my grad school days, we’d order reagents and hope nothing had shifted between catalogs—sometimes ending up with twice-protected piperazines or another isomer altogether. Misidentified chemicals didn’t just mess up reactions. They could cost a week in repeat syntheses, not to mention the cost of wasted reagents. After that, we swore by CAS numbers. Shortcuts brought headaches. Chemists who ignore this end up telling stories about ruined experiments more than they care to remember.
Pharmaceutical labs can’t afford to play guessing games. A single mix-up translates to wasted batches, unhappy clients, and angry bosses. Regulations expect precision. One wrong digit on a label can mean scrapping a whole day’s work. More than a random string, 57260-72-7 prevents shipment mistakes and regulatory mishaps. For vendors selling globally, matching up local synonyms gets tricky. CAS numbers slice straight through translation slip-ups.
A lot of online marketplaces push purity levels, but smart buyers check the CAS. The chemical’s structure, its supply risk, even its current regulatory standing—every bit hinges on the correct number. Some customers ask about toxicity or legal issues, which also link back to the number, not the name. Lists from the European Chemicals Agency and similar authorities all go by CAS. Bad actors can’t skirt safety laws by slapping on a new alias: the number brings up the same restrictions every time.
There’s never a guarantee against accidents, but using the right CAS number shaves off most common errors. Label bottles clearly, buy reagents only after confirming matches, and keep a searchable record of what’s in the cupboards. Digital inventories transform from fluffy bureaucracy into lifesavers whenever audits or scale-ups happen. Some labs take things a step further. If a new recruit doesn’t know the CAS of something they’re working with, a quick quiz fixes that knowledge gap fast. It isn’t hazing, just basic safety.
For students and pros, remembering 57260-72-7 opens doors in literature searches, helps spot reviews, and links patents. It feels like a small step, but so much research pivots on getting these little details correct. The CAS number might not look meaningful, but it’s the key that turns sheer information into real results.
I’ve always found the world of chemistry full of odd-sounding names, but behind each tongue-twister is a role that shapes what lands on pharmacy shelves or even in your favorite chemical experiments. 1-N-Boc-Piperazine isn’t something you’ll find mentioned over coffee, but it quietly pulls a lot of strings in the lab.
The pharmaceutical industry runs on clever building blocks. 1-N-Boc-Piperazine fits that bill. Chemists use it to set up the foundation for medicines, especially for making antihistamines, antipsychotics, and drugs that aim at severe mental health issues or persistent pain. These drugs often share structures, and this compound adds flexibility for changing or finessing those formulas. Medicinal chemists rely on 1-N-Boc-Piperazine for its easy-to-handle protective quality, which means the rest of a molecule can develop without unwanted reactions. It isn’t the headline act—more the sturdy scaffolding holding everything steady during construction.
Before any pill hits the market, it’s gone through endless steps. One key trick involves making sure delicate groups stay safe while the rest of the molecule takes shape. That’s where the “Boc” part of the name comes into play—the Boc group locks in one end of the piperazine, letting chemists poke and prod the other side. Once everyone’s finished, they snap off the cap, revealing a ready-to-go piperazine building block. This reliable technique shows up whenever piperazine-based molecules come under the microscope, whether that leads to cancer drugs or treatments for infections.
Drug companies don’t get all the fun. Universities and research teams treat 1-N-Boc-Piperazine as a trusty base for making new materials, especially polymers or advanced coatings. The protected nitrogen atom makes this compound a safe staging area for inventing all sorts of odd and useful materials. Sometimes that means experimenting with substances that could store energy better or offer a smarter way to clean up pollution. The flexible chemistry lets researchers push boundaries without running into a dead end after one or two tweaks.
Given its heavy use, sourcing worries pop up now and then. Pharmaceutical supply chains sometimes have hiccups, which ripple into research labs as well. If a lab can’t get enough of a core ingredient, timelines stretch and costs climb. From my perspective, broadening supplier pools and encouraging investment in local manufacturing help dodge most disasters. Another fix involves training more students in hands-on synthesis, which boosts problem-solving chances when something goes awry in production. The broad takeaway: don’t put all your eggs in one basket, and be sure there are enough folks who can rebuild the basket if it snaps.
I’ve seen both chemistry students and seasoned researchers gravitate to 1-N-Boc-Piperazine for one simple reason—it works. It doesn’t win awards for flashiness, but its design and reliability have shaped countless innovations in health and science. People may not realize a bland-sounding ingredient could quietly steer whole branches of research, but ask any medicinal chemist about the best tools on their bench, and you’ll hear a story or two about this compound’s essential role.
Chemists searching for 1-N-Boc-piperazine rarely care about tiny glass bottles. For folks working in pharmaceuticals, specialty chemical synthesis, or even contract manufacturing, access to large amounts is what counts. Lab-scale availability often feels like a given—we’re used to stocking 5 or 10 grams without a sweat—but the situation shifts once companies want to move past research and start scaling up. Suddenly, sourcing means supply chains, customs paperwork, batch consistency, and price negotiations.
Anyone involved in medicinal chemistry recognizes the building block status of piperazine derivatives. Masking its amine group with Boc protection, like with 1-N-Boc-piperazine, offers synthetic flexibility—helpful for making new drugs without side reactions. Those not bogged down in synthesis may wonder if it’s just another name in the catalog, but for process chemists and commercial buyers, its stable, protected form lets them tackle complex projects by the kilo.
Once quantity grows, price per gram shrinks. That’s where differences among suppliers become blindingly clear. I’ve seen companies request 50 kilograms and get blank stares from domestic reps. Some firms in Europe or North America might quote, but often can’t keep up with volume, or offer lead times that don’t help a project on the clock. China and India step in here. Their chemical manufacturing infrastructure and raw material sources can put big drums of material on a loading dock faster than local specialty shops even reply to emails.
Rapid industrialization changed the picture, but not everyone in the West feels comfortable buying direct from overseas. There’s always the worry about inconsistent quality or regulatory headaches. Long gone are the days when chemists just accepted any powder as long as it passed HNMR. Auditing facilities, checking for GMP compliance, and passing customs—these headaches sit close to every scale-up project.
Plenty of people take a “COA looks good, let’s buy” approach, treating the Certificate of Analysis as gospel. In reality, bulk batches occasionally differ. Over the years, I’ve watched contractors fight delays because one drum met the paperwork but failed the in-house check.
Smart teams order a small test batch first, run their own analytics, and only then approve bigger lots. Getting an independent lab involved before full payment isn’t just bureaucracy—it’s risk management. Every project manager learns the sting of underestimating quality hiccups when a deadline looms. NMR, HPLC, and moisture check can save weeks of lost time.
We’re seeing more manufacturers step up, invest in QA, and seek certifications. A few now guarantee lot-to-lot consistency and offer technical support that used to be rare in chemical distribution. These businesses know that reputations build slowly and vanish overnight if something goes wrong on a client’s reactor.
As environmental concerns grow, companies want more information about how their chemicals are made. Workers in compliance and sustainability check that their partners avoid pollution-heavy processes or provide proper documentation. Transparency and accountability improve trust between buyers and sellers, and that trust matters as much as the purity numbers on a product sheet.
Access to bulk 1-N-Boc-piperazine opens doors for drug makers, biotech startups, and chemical engineers with ambitious projects. Sourcing isn’t a simple button click, especially at large scales. Chemists and supply managers weigh supplier reputation against price and regulatory safety nets. Lessons learned from failed batches or long delays stick around, and teams grow smarter with each challenge. In the end, finding the right partner for reliable bulk chemicals often shapes what gets made—and who succeeds—in the lab and beyond.
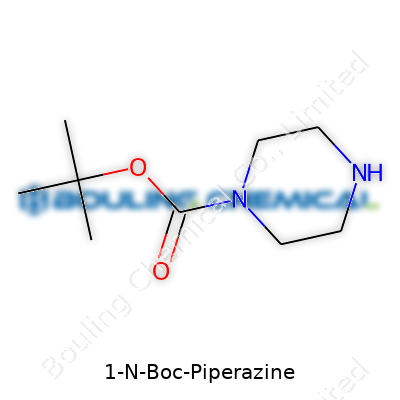

| Names | |
| Preferred IUPAC name | tert-butyl 4-piperazinecarboxylate |
| Other names |
Boc-piperazine 1-tert-Butoxycarbonylpiperazine N-Boc-piperazine tert-Butyl piperazine-1-carboxylate |
| Pronunciation | /ˈwʌn ɛn bɒk paɪpəˌreɪziːn/ |
| Identifiers | |
| CAS Number | 4972-31-0 |
| 3D model (JSmol) | `3d:7l1e4yl7k1ytnl9ayd6d5lo5wla40` |
| Beilstein Reference | 1202318 |
| ChEBI | CHEBI:131122 |
| ChEMBL | CHEMBL2059871 |
| ChemSpider | 143911 |
| DrugBank | DB13752 |
| ECHA InfoCard | EC number: 618-574-0 |
| EC Number | 603-203-00-7 |
| Gmelin Reference | 1071451 |
| KEGG | C14325 |
| MeSH | D010924 |
| PubChem CID | 10116 |
| RTECS number | TZ3910000 |
| UNII | L33TLB86B9 |
| UN number | UN3276 |
| Properties | |
| Chemical formula | C9H18N2O2 |
| Molar mass | 202.28 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.1 g/cm3 |
| Solubility in water | Slightly soluble |
| log P | -0.02 |
| Vapor pressure | 0.0126 mmHg at 25°C |
| Acidity (pKa) | 9.8 |
| Basicity (pKb) | 3.8 |
| Magnetic susceptibility (χ) | -75.4e-6 cm^3/mol |
| Refractive index (nD) | 1.488 |
| Viscosity | Viscous oil |
| Dipole moment | 2.32 D |
| Thermochemistry | |
| Std enthalpy of formation (ΔfH⦵298) | -499.7 kJ/mol |
| Pharmacology | |
| ATC code | |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS07, Exclamation mark |
| Pictograms | GHS06, GHS08 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | P264, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | > 109.3 °C |
| NIOSH | Not established |
| PEL (Permissible) | No PEL established. |
| REL (Recommended) | 1-10 |
| Related compounds | |
| Related compounds |
Piperazine N-Methylpiperazine 1-Boc-4-methylpiperazine 1-Boc-4-fluoropiperazine 1-Boc-3-piperazinecarboxylic acid N-Boc-piperidine |