Chemistry keeps finding new uses for heterocyclic compounds, and 1-Methylpyrrolidin-3-ol is one of those stories. This molecule, built around a pyrrolidine ring with a methyl group on the nitrogen and a hydroxyl group on the third carbon, started getting attention in the latter half of the 20th century as interest in fine-tuned solvents and drug design increased. Early accounts grew out of pharmaceutical synthesis labs, where researchers tinkered with ring modifications to find new routes to pharmaceuticals and fine chemicals with improved profiles. Synthetic routes evolved along with a deeper understanding of pyrrolidine chemistry, leading to the kind of access we have today—usually in small batches for research, but occasionally at scale for specialty applications.
1-Methylpyrrolidin-3-ol doesn’t get the same spotlight as its famous relatives like pyrrolidine or its derivate, N-methylpyrrolidone (NMP). Still, as a building block, it brings both a polar hydroxyl group and an N-methyl group to the table. Think about the way certain plastics, agrochemicals, and drug intermediates rely on niche molecules that bridge gaps in reactivity. This substance helps bridge solubility and reactivity trends across categories. Available commercially as a colorless to slightly yellow liquid, the chemical has found use in labs seeking either to tweak its nucleus or put it to work as an intermediate in synthesis routes.
You can pick out 1-Methylpyrrolidin-3-ol by its slightly amine-like odor and viscous liquid form at room temperature. Boiling point lands well above water, often in the range of 200–230°C, telling you it sticks around in most reactions without evaporating away. The presence of both a nitrogen and oxygen atom in its ring lends extra polarity; it dissolves well in water and alcohols, and acts as a reasonable organic solvent for certain reactions. Density settles close to 1.0 g/cm³, making measurements in the lab relatively straightforward, and its refractive index places it with related small amines. You get a slight basicity thanks to the nitrogen, though that’s tempered by the alcohol function which adds some hydrogen bonding capacity and can modify solubility profiles in mixed solvent systems.
Suppliers commonly provide 1-Methylpyrrolidin-3-ol at purities above 98%, usually confirmed through nuclear magnetic resonance (NMR) or gas chromatography. Labels cover the basics: product name, chemical formula (C5H11NO), and batch-specific data for quality control. IUPAC names matter for regulatory paperwork, though you’ll also see common names and catalog references. Storage conditions focus on keeping the container tightly sealed, away from oxidizers and direct sunlight, and usually recommend a well-ventilated, cool, dry place. Safety Data Sheets (SDS) outline hazard codes and harmonized signal words, so anyone working with this molecule gets a clear run-down of handling procedures and first aid.
Laboratory synthesis often starts with pyrrolidine or its derivatives, altering the ring nitrogen with a methyl group through alkylation. Then, a selective oxidation or hydroxyalkylation at the third carbon creates the alcohol. In practice, traditional organic chemistry tools step into play—classical oxidants like potassium permanganate or chromium trioxide, or modern catalytic systems that promise lower waste and milder conditions. Reaction design here centers on selective activation and protection strategies, since over-oxidation or unwanted ring opening can spoil the batch. Skilled chemists know purification typically follows, with distillation under reduced pressure or chromatographic separation rounding out the process. Experience helps, because yields fluctuate depending on control over stoichiometry and temperature.
The two key functional groups in 1-Methylpyrrolidin-3-ol—the secondary amine and the alcohol—open up plenty of chemistry. The hydroxyl group gets esterified or oxidized to a ketone, while the nitrogen gets quaternized for surfactant synthesis or alkylated toward specialty quaternary compounds. Cross-coupling reactions make use of the molecule’s nucleophilicity, and the alcohol function becomes a handle for modification in pharmaceutical lead generation. Interstate chemistry between the nitrogen and oxygen positions, including for cyclization or ring expansion, makes this a flexible starting material. Real examples include the use of this molecule to build more complicated spiro-fused structures or chiral ligands for metal-catalyzed reactions, an area where new chemical technologies often start.
You might run into 1-Methylpyrrolidin-3-ol under names like N-Methyl-3-hydroxypyrrolidine, 1-Methyl-3-pyrrolidinol, and sometimes short-form catalog abbreviations like MPNO. The IUPAC and CAS (616-29-5, typically) references show up on technical documents, and some commercial suppliers have their own trade names. Across languages, naming conventions tend to follow the N-methyl substitution and the alcohol position. In research circles, shorthand often gets used; accurate labeling in storage matters, since mix-ups with similarly named analogs can cause trouble in sensitive syntheses.
The core of safe handling boils down to chemical hygiene and personal protective equipment (PPE): gloves, goggles, and lab coats. 1-Methylpyrrolidin-3-ol carries mild irritation risks for skin and eyes. Organic vapor filters and fume hoods come standard in any lab dealing with amines, as odors may linger and minor vaporization can sensitize some users over time. Spill management means absorbent pads for small releases and well-ventilated cleanup for bigger spills. Waste gets segregated as organic material, never poured down the drain. Exposure limits remain under continued investigation, but the industry tends to default to the stricter side when in doubt. Emergency guidelines cover basic chemical exposure: flush skin with water, seek fresh air for inhalation, chemical removal for eyes, and rapid medical evaluation if symptoms persist or systemic exposure is suspected.
Industry uses for 1-Methylpyrrolidin-3-ol have focused on its midsize, versatile chemical structure. Pharmaceutical development scans this molecule for roles in precursor synthesis and as a chiral auxiliary in asymmetric transformations. Certain agrochemical pathways benefit from its solubility traits, and some materials chemists look at it for specialized resin modification or as a linker in polymer construction. The polar nature of the structure also draws interest for solvent systems, where it fits in at the margins, improving mixing or helping dissolve polar and nonpolar molecules. My experience working with pharmaceutical startups has shown that compounds like this often serve as intermediate tools—not the final drug, but important puzzle pieces in getting synthesis steps to align. Research applications dive deeper into novel scaffolds, as small modifications to this base structure can lead to wide changes in bioactivity or material properties.
Current research chases improvements in both synthetic efficiency and environmental impact. There’s a push toward milder, more sustainable oxidations, and routes that avoid wasteful solvents or hazardous reagents. Some groups dig into enantioselective synthesis, looking to generate chiral versions of the molecule for use in asymmetric synthesis, which pays off in drug discovery. Recent literature explores functionalization at other points on the ring, or modifications to build heterocyclic libraries for screening against new targets in medicinal chemistry. Collaboration across research institutes and chemical suppliers has made high-purity samples easier to access, and advanced analytical techniques like high-resolution NMR and mass spectrometry bring a new level of insight into reaction optimization. Patent activity sometimes clips up on unexpected pharmaceutical discoveries, with new analogs stemming from this versatile platform.
Studies on 1-Methylpyrrolidin-3-ol’s toxicity keep growing, as regulators and health professionals try to get ahead of any long-term use risks. Acute exposure tracking looks for effects on the nervous system, as related pyrrolidines have shown activity as enzyme inhibitors in animal models. Chronic studies follow metrics like liver and kidney function with repeated dosing, though conclusive data in humans remain limited. In vitro results suggest low toxicity at the concentrations typically handled in labs, but animal models help flag any potential for accumulation or unforeseen metabolic byproducts. Handling guidelines echo what I learned in chemical safety training: err on the side of caution and get updated data sheets before scaling up. The compound does not show significant carcinogenic, mutagenic, or reproductive toxicity at reported exposure levels, but wider studies will tell the full story as research use spreads.
Innovation keeps bringing molecules like 1-Methylpyrrolidin-3-ol into the spotlight. Cleaner syntheses and improved functionalization strategies should drop production costs, opening the door for wider testing as a pharmaceutical intermediate or specialty solvent. Researchers are pursuing biocatalytic processes, leveraging genetically engineered enzymes to build this compound with fewer steps and less environmental impact. Expanded toxicological profiles will support its adoption in regulated settings, from advanced material manufacturing to life sciences. My time in the lab has proven that once a molecule proves useful and safe, industries find creative ways to integrate it. With the future of green chemistry and precision pharmaceuticals relying on specialized building blocks, 1-Methylpyrrolidin-3-ol stands as a compelling candidate for further development and real-world application.
1-Methylpyrrolidin-3-ol isn't the kind of compound most people casually mention, but it quietly takes its place in laboratories and product development. Chemists and researchers know it by the backbone of its structure: a pyrrolidine ring with a methyl and hydroxy group. These little tweaks matter a lot in chemistry because small changes in molecular structure spark big shifts in reactivity and possible uses.
Lots of specialty chemicals land in a dusty bottle on a shelf, but this one actually finds use. The most notable application of 1-Methylpyrrolidin-3-ol shows up as an intermediate. In practical terms, it acts like a building block for synthesizing more complex molecules. Many pharmaceuticals need these sorts of intermediates. Drug discovery usually calls for a wide variety of molecular options, and this compound helps fill out that catalog because it's both reactive and adaptable.
Fine chemical production also depends on intermediates like this. Creating agricultural chemicals, such as pesticides or herbicides, starts with finding the right backbone molecule, and researchers often select small pyrrolidine-related structures for their balance of stability and reactivity. If a company develops a new active ingredient for crop protection, chemists might trace the synthetic chain right back to 1-Methylpyrrolidin-3-ol. I’ve chatted with lab techs who say it's surprisingly common for such molecules to end up in the pipeline of seed coatings, fungicides, or even vitamins.
Chemistry companies don’t just pick random molecules for their processes. They need something reliable. This compound dissolves in water and some common solvents, which makes it versatile for different reactions. It also holds promise in developing new compounds for research and testing—think of it as a square peg that fits enough round holes to matter. It's not every day you come across something that plays nicely with tough chemical reactions and keeps its properties consistent.
Besides medicine and farming, you’ll find this molecule working its way into the research end of specialty coatings and even some adhesives. Sourcing reliable intermediates means companies save time and resources, which means more competitive pricing for the end customer. I’ve seen teams leverage this in accelerating projects that otherwise might bog down with less cooperative reactants.
No chemical is perfect, and 1-Methylpyrrolidin-3-ol presents some classic lab hazards. Like many small organic compounds, it can cause irritation and isn’t meant for direct human contact. The Material Safety Data Sheet lists gloves and goggles as essential. Nobody in the know takes these warnings lightly because the cost of cleaning up accidents far outweighs extra caution. Responsible labs and manufacturing sites keep tight tabs on storage and disposal, not just for legal reasons, but because it’s the right thing for workers and the environment. Smart practices and a bit of common sense go a long way.
Every time a new compound gets considered for large-scale use, the same questions come up about sustainability and safety. Chemists and manufacturers know that the industry needs to cut down on waste and use greener processes. One solution involves recycling solvents and finding less harmful alternatives. Some labs explore biocatalysts to replace harsher chemical steps, but not every process swaps out the old for the new right away. By focusing on efficiency and ongoing safety upgrades, companies aim to keep improving both the environmental impact and reliability of chemicals like 1-Methylpyrrolidin-3-ol. With stricter oversight and curiosity driving innovation, the chemical industry continues to modernize far beyond the era of careless waste.
Stepping into a chemistry lab brings a mix of excitement and caution. I still remember my early days, gloves too big, goggles fogging up, fumbling with pipettes. No matter how confident you feel, new solvents and reagents demand respect. 1-Methylpyrrolidin-3-Ol does not hit the daily news, but behind closed lab doors, people work with it and know things can turn dicey quickly without solid habits.
Chemical names rarely do justice to how a substance behaves. This compound can irritate skin, eyes, and the respiratory tract. Spills or splashes can happen fast, especially if you’re rushing or distracted. Inhalation causes headaches or worse; accidental contact burns. Stories I’ve heard are not pretty—red skin, watery eyes, trips to the doctor that ruined someone’s week. The importance of reviewing material safety data sheets goes beyond bureaucracy; these documents share real, tested warnings and first aid advice.
Gloves matter more than you think. Nitrile ones, double layered if you’re moving more than a drop. Chemical splash goggles cover more skin than simple safety glasses—your eyes deserve that extra protection. A buttoned-up lab coat goes a long way. One time, I relied on a thin cotton shirt and paid for it with a red mark on my arm that stuck around after a small spill.
Good habits include tying back long hair, avoiding baggy sleeves, and checking glove integrity before and after use. Watch where your hands go. Rushing cleanup? That’s when the worst slips happen.
With solvents and volatile chemicals, working in a fume hood cuts risk by half or more. Breathing in fumes, even at low levels, harms over time. If you notice a strong odor, chances are the exposure is too high. Modern fume hoods pull vapors away and shield you in case of a splash or minor explosion. I’ve seen colleagues skip this step for “just a quick transfer” and pay for it by feeling dizzy all afternoon.
This chemical shouldn’t sit next to acids, strong oxidizers, or ignition sources. Flammable liquids cabinet with clear labeling does the trick. Keep the bottle closed tight. Big, bold labels save confusion. I've witnessed frantic searches for “where did we put it?” in the middle of urgent experiments—labeling avoids these moments.
Spills happen to the most careful among us. Have spill kits ready—absorbent pads, neutralizers, and a plan for disposal. Paper towels won’t cut it. Alert your colleagues; don’t try to tough it out alone or cover it up. Every experienced tech knows cleaning up quietly turns small mistakes into bigger disasters. Immediate skin exposure? Head to the eyewash or safety shower and do not wait.
Reading guidelines once helps. Practicing procedures during low-stress moments saves you when pressure ramps up. Supervisors and mentors set the tone; they shape how careful newcomers act. Clear, ongoing instruction builds a safer space for everyone.
Safety can sound like extra work, but shortcuts don’t pay in the long run. Using the right gear, labeling storage, maintaining clear workspaces, and sticking to proper ventilation should be a regular drill. Staying honest about risks, learning from near misses, and teaching newcomers reinforces healthier labs. Nobody wants to add their name to the list of cautionary tales in lab safety meetings—or worse, end up in the emergency room.
A name like 1-Methylpyrrolidin-3-Ol might sound intimidating, especially if you don’t work in a chemistry lab every day. Truth is, compounds like this shape everything from new medicines to safer chemicals in our everyday products. This one belongs to a chemical family known as pyrrolidines. Its structure centers around a five-membered ring with four carbon atoms and one nitrogen. Attaching a methyl group (-CH3) at the first carbon and a hydroxyl group (-OH) at the third makes its chemistry distinctive.
The molecular formula of 1-Methylpyrrolidin-3-Ol is C5H11NO. Looking at its skeletal formula, you find a five-member ring (the pyrrolidine), where the nitrogen sits at one corner. Methyl sits on the nitrogen atom, and a -OH group sits at the third carbon. Chemically, this seems straightforward, but every switch in a molecule’s architecture opens doors to different behaviors in real life.
People sometimes ask why chemists care about exact structures. From my experience as a science communicator, even small changes can make a world of difference. Different attachments can mean the difference between a molecule used for flavor and one flagged as toxic. With 1-Methylpyrrolidin-3-Ol or its cousins, small substitutions decide how well the molecule binds to biological targets, how soluble it stays in water, or whether it passes through the human body safely.
The hydroxyl group provides polarity, making the molecule more at home in water-based environments. That influences industrial applications and the pharmacological journey inside a body. The methyl group bolsters some stability and sometimes tweaks the way a compound gets metabolized. Chemists map these subtle changes to predict whether a candidate fits as a drug or a specialty solvent.
Researchers look at pyrrolidine derivatives for their building-block power in pharmaceuticals and materials science. They serve as starting points for more complicated molecules — think synthetic routes toward active pharmaceutical ingredients or green alternatives for outdated solvent systems. In practical settings, professionals debate not just usefulness, but how easily and safely these chemicals can be produced.
Safety standards shape discussions around solvents and drug intermediates. History shows that even modest alterations in structure can result in different, sometimes unexpected toxicities. For instance, a simple methyl or hydroxyl next to a nitrogen can affect how a molecule breaks down in the liver or interacts with proteins. That’s why regulatory scrutiny, peer review, and methodical laboratory tests always follow the introduction of these substances to any process where people could be exposed.
The challenge isn’t just creating molecules like 1-Methylpyrrolidin-3-Ol—it’s making sure their real-world use stays responsible. Transparent data sharing, cross-checking chemical properties, and investing in further safety studies create trust between scientists, businesses, and the public. With interdisciplinary teams—including toxicologists, synthetic chemists, and physicians—every step in a compound’s life story gets a second look.
In practical terms, researchers continue to refine how these chemicals are made, both to cut waste and to protect workers from unintentional exposure. Laboratories now lean on green chemistry principles. Lowering reaction temperatures, using less toxic reagents, and recycling solvents reduce environmental impact. These approaches ensure society enjoys advances in chemistry without putting our health or surroundings at risk.
1-Methylpyrrolidin-3-Ol shows up in labs and some manufacturing sites because chemists rely on its unique structure. If you’re handling this compound, you’ll want to keep more than just basic storage tips in mind. This isn’t a bottle of hydrogen peroxide you keep under the sink; its chemistry means careless storage carries real risks.
Run-of-the-mill overlook turns into a disaster with chemicals like this. I’ve walked into labs where someone shoved every solvent, acid, and base on a single wobbly shelf near the window, convinced proximity saved time. What happens in that setup when sunlight hits a volatile compound? Spoilage and spills become real threats. Temperature swings might throw a wrench in your research, or worse, threaten safety.
There’s also the risk of contamination. Air or moisture can sneak into poorly sealed bottles, changing the character of the material. In a precision-driven environment, that sort of compromise can lead to failed experiments and frustration. Spill clean-up in a poorly ventilated closet brings its own headaches, both for health and regulatory compliance.
Good habits start with the right container. Always use tightly sealed, chemical-resistant bottles. Polyethylene bottles or amber glass stand up to the demands of 1-Methylpyrrolidin-3-Ol—never grab an old soda bottle or cheap, thin plastic from the discount bin. Labels need to be clear, detailed, and include hazard information you can spot instantly, even in a panic.
Pick a spot that stays cool and shields the contents from direct light. Room temperature usually suffices, as long as it doesn’t spike or plummet on a whim—think steady, basement-lab vibes rather than sunblasted attic shelves. For extra sensitive operations, a temperature-monitored cabinet takes the guesswork out of it.
Never store this material with acids or oxidizers. I once heard about a neighboring lab that stuck a faulty bottle of solvent next to a strong oxidizer—an avoidable incident left their team scrambling in the aftermath of a minor explosion. Maintaining a chemical logbook serves not just the compliance team but everyone who works with you; tracing sources of contamination or waste gets a whole lot easier.
Ventilation deserves respect. Fumes from organic chemicals don’t play nice—strong airflow from a chemical safety cabinet keeps vapors away from ignition sources and noses alike. If you need to move the bottle, double-check the seal and wear gloves plus goggles. Even brief exposure can irritate skin or eyes.
Training remains non-negotiable. Newcomers should walk through storage protocols alongside experienced staff instead of diving in blind. In my own experience, labs that schedule regular safety reviews see fewer accidents and less wasted material. In a pinch, a well-trained team member can prevent a small mistake from becoming a full-blown crisis.
Respect for each material builds over time. Giving 1-Methylpyrrolidin-3-Ol the attention it demands safeguards not just workflows, but your colleagues and investment in research. Old hands in the lab appreciate those who treat every storage decision as part of a bigger safety puzzle. That attention pays off every single day.
1-Methylpyrrolidin-3-ol comes from a chemical family that scientists and industrial workers often cross paths with. Chemists use similar molecules in labs and factories thanks to their handy solvent power or as part of synthesizing other materials. Curiosity usually arises over substances like this because they fuel progress, but not every chemical deserves a spot on anyone’s favorite list. The flip side gets real when toxicology pulls up a seat at the table.
My time working in labs taught me to read the label twice and keep an eye on hazard sheets. Many chemicals that don’t sound alarming can turn out to be surprisingly tough on health. Put your hands near a bottle of 1-Methylpyrrolidin-3-ol and the nose quickly picks up a pungent whiff. A closer look at its profile shows that similar pyrrolidines often cause skin, eye, or respiratory irritation. Ingesting or inhaling these compounds—even a small amount—can set off headaches, nausea, or dizziness. Extended skin contact sometimes brings rashes or a slow-burning soreness that pushes you to the sink to scrub, rinse, and regret not wearing gloves sooner.
Folks like to ask, What happens if it’s around for the long haul? Experience with related chemicals offers clues. Chronic exposure can mean trouble: potential damage to organs, especially the liver and kidneys, crops up in animal studies of this chemical family. The scientific literature doesn’t give 1-Methylpyrrolidin-3-ol a free pass; researchers urge caution due to possible nervous system effects if inhaled or absorbed often. One study from the National Institute for Occupational Safety and Health linked related solvents to changes in mood and sleep patterns. None of that spells confidence for daily handling without protection.
Lab veterans don’t treat small molecules lightly. My own rules: keep fume hoods on and eye-wash stations close. Gloves and goggles skip the fashion contest, but everyone realizes it beats the hassle of a chemical injury. Industrial guidelines push for closed systems and personal protective equipment. Take the story of a technician I worked with—one hurried transfer without proper safety glasses led to a trip to occupational health and weeks of blurry vision.
OSHA and the European Chemicals Agency keep reviewing chemicals like 1-Methylpyrrolidin-3-ol to set boundaries on exposure. Nobody gets nostalgic for the days before safety data sheets were standard. Training makes the biggest difference—once, after a session on pyrrolidine risks, new interns stopped cutting corners and started logging every splash and fume. If accidents happen, protocols call for thorough washing, reporting, and, sometimes, medical check-ups.
Companies with an eye on worker safety and product quality look for greener alternatives wherever they can. In some processes, swapping out risky solvents pays back in less liability and better morale. From my own experience, flagging a hazardous chemical often leads to brainstorming safer recipes or engineering upgrades that decrease handling time and direct contact. Asking tough questions about new chemicals before adoption keeps risk low and trust high.
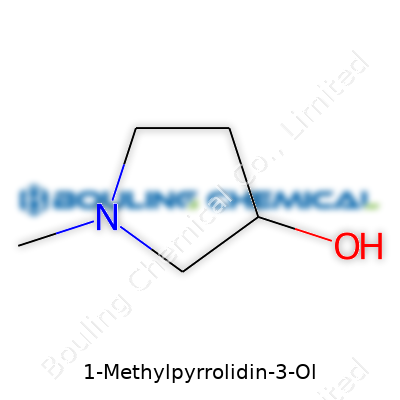
| Names | |
| Preferred IUPAC name | 3-Hydroxy-1-methylpyrrolidine |
| Other names |
1-Methyl-3-hydroxypyrrolidine 1-Methyl-3-pyrrolidinol |
| Pronunciation | /waɪˈmɛθɪl pɪˌrɒlɪˈdiːn θri ɒl/ |
| Identifiers | |
| CAS Number | 6602-33-9 |
| 3D model (JSmol) | ``` g003200 ``` |
| Beilstein Reference | **906595** |
| ChEBI | CHEBI:189445 |
| ChEMBL | CHEMBL1490627 |
| ChemSpider | 31113 |
| DrugBank | DB02307 |
| ECHA InfoCard | 100_123_646 |
| EC Number | 83985-01-5 |
| Gmelin Reference | 1861310 |
| KEGG | C18915 |
| MeSH | D021277 |
| PubChem CID | 10471496 |
| RTECS number | UQ2975000 |
| UNII | IA91L1OD44 |
| UN number | UN3439 |
| CompTox Dashboard (EPA) | urn:uuid:2cb31beb-472f-4deb-9e4b-0719932ed18e |
| Properties | |
| Chemical formula | C5H11NO |
| Molar mass | 101.16 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | Amine-like |
| Density | 0.997 g/cm³ |
| Solubility in water | miscible |
| log P | 0.02 |
| Vapor pressure | 0.472 mmHg at 25 °C |
| Acidity (pKa) | 18.7 |
| Basicity (pKb) | pKb = 4.44 |
| Magnetic susceptibility (χ) | -43.59·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.463 |
| Viscosity | 0.945 cP |
| Dipole moment | 2.16 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 312.9 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -198.5 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | “-3940 kJ/mol” |
| Pharmacology | |
| ATC code | N07XX |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319 |
| Precautionary statements | P261, P264, P271, P272, P280, P302+P352, P321, P362+P364, P501 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 108.4 °C |
| Autoignition temperature | 215 °C |
| Lethal dose or concentration | Lethal Dose or Concentration (LD50/LC50): "LD50 (oral, rat): 970 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 650 mg/kg |
| NIOSH | RN/105134-97-2 |
| PEL (Permissible) | PEL (Permissible exposure limit) for 1-Methylpyrrolidin-3-ol is not specifically established by OSHA. |
| REL (Recommended) | REL (Recommended Exposure Limit) for 1-Methylpyrrolidin-3-ol: "No specific REL established |
| Related compounds | |
| Related compounds |
Pyrrolidine 1-Methylpyrrolidine 3-Pyrrolidinol N-Methylpyrrolidine 1-Methyl-3-pyrrolidone |