Organic chemistry, as a field, owes a lot to the study of five-membered heterocycles like pyrroles. Chemists started tinkering with these compounds in the late 1800s, propelled by natural products, pharmaceuticals, and the hunt for new dyes. 1-Methylpyrrole came into the limelight as researchers discovered the power of methylation—something as simple as adding a methyl group to the nitrogen in pyrrole opens up a world of new chemical behaviors. My own encounters with textbook reactions showed me how easily methyl-substituted pyrroles could slip into synthesis plans, especially for complex pharmaceuticals and material science projects. The path that brought 1-methylpyrrole to laboratory shelves isn't just about incremental science—it tracks with society's demand for better drugs, stronger polymers, and richer dyes.
Anyone walking through a lab supply catalog bumps into 1-methylpyrrole somewhere between the fancier reagents and the industrial mainstays. It's a clear, colorless to pale yellow liquid, pungent but manageable compared to its analogs. The molecule's N-methyl group really makes a difference—it blocks some reactions you’d expect with plain pyrrole, but also nudges new possibilities. Production volumes aren’t high compared to monomers or solvents, but specialty chemical buyers know it’s a staple for synthesis, especially in agrochemicals and pharmaceuticals. The cost stays reasonable, reflecting its relative accessibility and steady—but not explosive—demand.
The physical properties show the subtle changes that methylation brings. 1-Methylpyrrole boils at roughly 110°C, which puts it a bit above pyrrole itself. It remains soluble in common organic solvents like ether, benzene, and chloroform. Chemists notice its stability: without the free pyrrolic hydrogen, the compound resists some of the tautomerism and air oxidation issues that trouble plain pyrrole, though it still doesn’t last forever on the bench. Its mild basicity stands out, too—not quite an amine, more nucleophilic than pyrrole, and ready to take part in all sorts of cycloaddition or substitution games. The molecule weighs just 81 grams per mole but packs a punch in reactivity.
On any bottle of 1-methylpyrrole in a regular research lab, labeling states its CAS number—96-43-5. Purity ranges between 97% and 99%, with trace water and secondary amines noted for anyone doing sensitive work. Most suppliers offer it in amber glass to minimize light exposure. You'll find hazard pictograms and warnings about skin and respiratory irritation. Shelf life and recommended storage—cool, dry, away from strong acids or oxidizers—should always be respected. From my own time as a lab manager, those little details on a reagent bottle made daily life safer, especially when students weren't familiar with the quirks of these volatile organics.
The textbook synthesis starts with pyrrole, treating it with methyl iodide under basic conditions. One-pot reactions rely on methylating agents like dimethyl sulfate or methyl tosylate, avoiding multi-step purification. Some companies pursue routes from 1-chloropyrrole using methyl magnesium bromide, especially for scale-up, since this approach trims byproducts and residual starting material. Distillation under reduced pressure polishes the product, stripping out high-boilers and securing the kind of purity demanded by pharma or electronics customers. The preparation reflects a deep understanding of practical organic synthesis—simple when done right, unforgiving if you cut corners.
Chemically, 1-methylpyrrole shows a nimble balance of reactivity. Since an N-methyl group blocks N-H reactions, chemists can direct transformations to the 2- or 3-position: Vilsmeier–Haack formylation, directed lithiation, and halogenation all proceed smoothly. You can't run Fischer indole syntheses or tidy condensation reactions like you might with unsubstituted pyrroles, but you gain the ability to survive harsher conditions and avoid polymerizing side reactions. As a nucleophile, 1-methylpyrrole still attacks electrophilic aromatic sites, bringing unique substitution patterns that get leveraged in fine chemicals and specialty pigment production. In the lab, these directed modifications illustrate the value of N-protection—not just for yield, but for unlocking totally new scaffolds.
You’ll spot 1-methylpyrrole marketed as N-methylpyrrole, Pyrrole, N-methyl-, or even 1H-Pyrrole, 1-methyl-. In literature, abbreviations like MePyr or NMPyr appear. Accurate naming matters a lot: order under the wrong synonym and you risk receiving the di- or trimethyl version, with totally different reactivity. Retailers often highlight alternate names to reduce confusion among international buyers, especially since CAS numbers and synonyms sometimes get tangled up in shipment or customs papers.
Anybody handling 1-methylpyrrole quickly learns the nose-wrinkling punch it carries—ventilation is non-negotiable. The chemical vapor can irritate mucous membranes, and prolonged skin exposure chafes. Standard PPE—gloves, goggles, and a lab coat—makes a real difference. Spills need prompt cleanup, as volatile organics don’t just evaporate; they linger in the workspace. The compound’s flash point sits at just above room temperature, so it can catch fire from a stray spark if you’re not careful. Waste disposal goes into halogenated organics or hazardous waste streams, no short-cutting to regular drains. The importance of routine safety briefings stands out: every year, near-misses happen less from outright carelessness than from underestimating how aggressive even small quantities can get.
The reach of 1-methylpyrrole snakes through several industries. In pharma, it serves as a building block for antipsychotic, antihypertensive, and antiviral drugs, because its backbone fits neatly into bioactive molecules. Agrichemical firms craft insecticides and herbicides starting with this scaffold, counting on its stability under field conditions. In the world of materials science, the compound helps stitch together specialty polymers—think conductive polymers or new generation OLED emitters. Its presence in the dye and pigment industry reflects the deep colors and stability that methylation brings to molecular frameworks. Every sector leans on this molecule for its blend of ruggedness and adaptability—without requiring vast production lines.
The R&D side of 1-methylpyrrole has moved well past simple synthesis. Labs worldwide now chase after functional derivatives—like halogenated, nitrated, or fused-ring versions—to tune everything from fluorescence to anti-tumor activity. Teams focus on sustainable green chemistry methods, aiming to cut waste and use milder methylating reagents. Academic literature tracks advances in catalyst design for selective functionalization, making it easier to graft new groups onto the pyrrole ring without wrecking the methyl unit. Every conference season reveals some new spin: photoredox catalysis, bio-orthogonal labeling, or all-organic battery ingredients. In my grad school days, exploring heterocycles meant constantly bumping into new and surprising uses for such a compact structure.
Toxicological research gives 1-methylpyrrole mixed reviews. Acute exposure tends to bring moderate irritation—nothing like the nastier benzene derivatives—but chronic data stays limited. Animal studies hint at low to moderate systemic toxicity, mostly at doses well above typical handling levels. A handful of published papers flag liver enzyme changes after prolonged high-dose exposure, but the data pool remains thin. Responsible use hinges on understanding these risks before they become headline fodder. Many safety teams want better long-term data, especially as applications expand into biomedicine; what works for a dye plant could stay worrisome in consumer goods or pharmaceuticals. As someone who’s spent time reviewing registers and SDS sheets, the value of real-world toxicity data cannot be overstated—it’s the difference between safe routine work and accidental mishaps.
The horizon for 1-methylpyrrole stretches wide, especially as synthetic routes get greener and industries demand smaller environmental footprints. Pharmaceutical chemists keep reaching for it to build complex drug candidates—a trend I saw even as an undergrad intern a decade ago. Materials scientists value its electron-rich aromaticity for organic electronics and sensors, fields that grow fast as flexible electronics edge closer to mass production. The dye sector, newly energized by sustainability rules, explores 1-methylpyrrole as a route to robust colors with less environmental baggage. Future developments may ride on bio-based starting materials or energy-saving catalytic systems, edging the process closer to the values that modern chemistry now prizes. Chemists will add more to the story—one methyl group, one new reaction, and one fresh application at a time.
Most people never hear about 1-Methylpyrrole unless they dig into technical journals or chemical catalogs. It’s easy to miss, hidden behind complicated names and formulas. Still, 1-Methylpyrrole shows up in far more places than you might guess, shaping the world around us in ways that go well beyond a laboratory shelf.
Ask anyone involved in making inks or specialty coatings, and you may get a knowing nod about methylpyrrole. Dye manufacturing leans heavily on this compound. 1-Methylpyrrole lets chemists start or tweak molecular skeletons that build up to bright, long-lasting pigments. Red, blue, or something in between, plenty of those intense colors on packaging and art supplies owe their stability and vibrancy to jobs done behind the scenes by 1-Methylpyrrole. Growing up, I never thought about how that comic book ink could stay crisp for years on a sunny shelf; it turns out, subtle backbone shifts in molecules, powered by this little compound, play a huge part.
Imagine turning a tiny molecule into a medicine that could save lives. Chemists constantly search for new building blocks to unlock medical breakthroughs. 1-Methylpyrrole starts off small but acts as a handy platform to build up much more complicated molecules, including antibiotics and anti-inflammatory drugs. Once, at a local pharmacy conference, someone compared these molecules to Lego blocks. If you use the right ones, you can snap together structures with targeted properties—stronger healing power or fewer side effects. 1-Methylpyrrole gives drug designers a key piece in that chemical toolkit.
Electronic gadgets rely on organic molecules to deliver performance—think OLED screens or specialty conductive materials. 1-Methylpyrrole’s structure allows engineers to develop new electronic materials that can flex, light up, or transport charge better than old-school alternatives. I met a grad student once who raved about its potential in flexible displays; his hands were always stained with strange colors from lab experiments. By tailoring organic electronics with compounds like this one, manufacturers can produce monitors that roll up or sensors printed right onto clothing—stuff that turns science fiction into reality.
1-Methylpyrrole’s job doesn’t end in research. Solvent manufacturers also tap into this compound, using it to dissolve or mix trickier ingredients. Some adhesives and cleaners need the unique properties this chemical offers—high performance under pressure, resistance to heat, and reliable mixing with other substances. Sourcing reliable materials matters a lot; during my stint helping a local factory, I saw production lines stall when a supplier changed their solvent mix, leading to days of troubleshooting. Subtle changes like swapping out a reliable compound can ripple across many fields, from automotive upkeep to electronics cleaning.
While 1-Methylpyrrole opens doors, its manufacturing and handling need real care. Safety regulations keep workers protected since exposure can lead to skin, eye, or respiratory irritation. Cutting corners in storage or waste management can backfire, both for workers and the environment. Shifting toward greener production methods stands out as one smart direction; catching pollution before it escapes the factory shows respect for both people and place, not just profit.
Chemicals that sound unfamiliar at first can still reshape entire industries. Watching 1-Methylpyrrole quietly power improvements in everything from ink cartridges to smartphone screens shows how deep curiosity and good design keep the world in motion. As more companies aim for cleaner, smarter chemistry, keeping an eye on compounds like this one may give us all a brighter, more sustainable future.
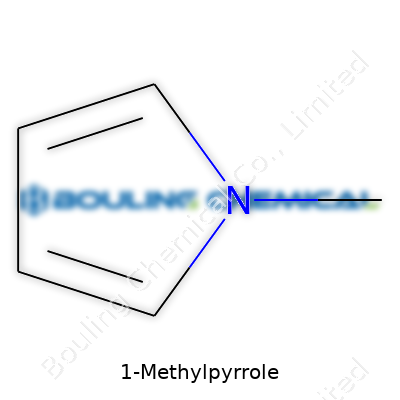
Every time someone asks about the chemical formula for something like 1-methylpyrrole, it unlocks more than just a line on a chalkboard. The formula gives direct insight into how a molecule behaves, where it fits in organic chemistry, and the impact it has outside the lab. In this case, 1-methylpyrrole has a formula of C5H7N. That simple string signals a lot for anyone who has played with these compounds or followed their story in science news.
Pyrrole itself lays the foundation—think of a five-membered ring, four carbons and a nitrogen. Slap a methyl group on the nitrogen, and you’ve just changed the rules: one small change, big impact. The molecular formula spells it out. Look closely: five carbons, seven hydrogens, one nitrogen. It’s a pattern that repeats not just on paper but in reaction flasks and in real-world materials.
The extra methyl group can change how the pyrrole ring interacts with other chemicals. Organic chemistry isn’t just a subject; it’s a world where tiny tweaks mean different properties—solubility shifts, reactivity takes a turn. I’ve watched students realize that a formula like C5H7N often points to more than a sum of its atoms. Add a methyl here, gain a new reaction intermediate there. Crafting drug molecules or putting together polymers doesn’t happen without paying attention to what’s on the label, and formulas like this one set the ground rules.
1-Methylpyrrole hasn’t stormed the headlines, but it plays into bigger stories—pharmaceuticals, organic electronics, even dyes. Chemists have looked to compounds like this one to nudge synthesis in the right direction, creating new building blocks or tweaking properties of final products. Each atom in C5H7N counts. It shapes boiling points, points the way toward how the compound acts in a mixture, or leads researchers toward alternative synthetic methods.
Over the years, seeing chemical names pop up in discussion often lead to quick searches for formulas, which anchor any meaningful talk about usefulness or hazards. Methylpyrroles, for example, turn up as intermediates. That means manufacturers or labs often keep an eye on purity and reactivity profiles, both of which hinge on knowing the correct formula. Slip up once and confusion follows—changing methyl placement changes everything, and so does misreading a chemical formula.
Mix-ups happen. More than once, I’ve seen folks grab the wrong pyrrole derivative and wonder why a reaction stalls or fails. Small differences in a formula create real-world headaches. Safety data, waste protocols, and inventory tracking all revolve around getting details right. With harsh or expensive chemicals involved, even a single-digit error could cost hundreds—or worse, lead to avoidable exposure hazards.
Chemical literacy isn’t just about reciting formulas like C5H7N. It’s about understanding what those numbers really mean in practice. Laboratories and companies need reliable shorthand to sort, label, and safely work with the mountain of organic compounds out there. Shortcuts and lazy habits, especially with naming and formula-writing, invite risk and confusion.
Teaching and learning about chemicals such as 1-methylpyrrole circles back to respect for accuracy. I’ve found that fostering curiosity—where formulas serve as a springboard for questions—opens the door to better science and, hopefully, safer work. The chemical formula matters, not just for exams or research papers, but for every measured scoop, every scaled-up synthesis, every new discovery in the world of molecules.
Anyone who’s spent time in a chemical lab learns pretty quickly that not all bottles on the shelf deserve the same treatment. Take 1-Methylpyrrole for example. Its low flash point means a single careless move can turn a tiny spill into a blazing headache. Storing this kind of solvent on a bench leaves you asking for trouble. At our lab, nobody ever parked these containers next to heat sources or under direct sunlight—too many stories float around about unexpected fires from folks who took shortcuts. Instead, the safest spot sits inside a well-ventilated flame-proof cabinet.
Letting vapors drift out isn’t a small issue with 1-Methylpyrrole. Once, a colleague ignored the state of a cracked sealing cap. The entire room started stinking in minutes, and she caught a pounding headache. Beyond the smell, repeated exposure takes a toll on your health. Keeping lids tight and always checking for leaks before putting anything away turns into a habit worth keeping. Plus, making a note of the open date on bottles helps people know just how old things are getting—no one wants to breathe fumes from a half-forgotten bottle.
Moisture throws things off in surprising ways. I learned the hard way after discovering a sticky mess in the bottom of a cardboard box where someone had thought a shelf in the janitor closet was good enough for storage. Humid rooms chip away at chemical purity. Even a little splash from mopping the floor can cause issues. Dry, cool storage limits these risks. Keeping temperatures pretty stable—ideally under 30°C—makes a big difference. Air conditioners aren’t there just for people, they help prevent chemical surprises too.
Sometimes the biggest risk comes from not knowing what’s in a container. Someone once left an unmarked flask in the common fridge. A visiting researcher took one sniff, thinking it was pure ethanol, and ended up with burning eyes. Bold, clear labels in permanent ink save everyone time and protect against mix-ups. Adding hazard symbols and the date for good measure puts the information where everyone can see it.
After a while, experienced workers develop a sixth sense for risky storage. Shortcuts cause accidents. Basic practices—like storing 1-Methylpyrrole away from acids, oxidizing agents, or open flames—add real protection. Good chemical hygiene also means routinely cleaning storage areas and making sure emergency spill kits are stocked. Accidents can and do happen, even to cautious folks. Training everyone in quick cleanup techniques keeps small spills from turning into disasters.
Most chemical storage rules seem obvious on paper until deadlines or distractions tempt people into breaking them. Sharing near-misses and lessons learned helps the whole team keep each other honest. Clear protocols, easy-to-find safety sheets, and routine checks all combine to keep 1-Methylpyrrole—and everyone handling it—out of danger. In the end, healthy respect for these substances comes down to practical habits and real teamwork.
1-Methylpyrrole comes with its own set of dangers. Along my days in the lab, I saw how it easily evaporates and lets off vapors known to sting the eyes and nose, even at low concentrations. If you’re not paying attention, a small spill or open vial can turn the room into a tough place to breathe. I’ve seen folks chase spills without gloves, only to find out later that it irritates the skin and leaves a smell that sticks for hours. Nobody enjoys that.
Dealing with chemicals like this makes you respect gloves and goggles. Splashing a bit of 1-Methylpyrrole on bare hands left one of my colleagues with red, itchy skin. The lab coat, gloves made with nitrile, and safety glasses aren’t just for comfort—they keep you from a nasty evening. Fumes drift quickly, so the fume hood becomes the best friend you have. Forgetting to use it just once will remind you never to skip it again. Respirators sit on the shelf for serious leaks, but most days, that hood pulls its weight.
Not every space can keep up with this solvent. The way it punches up a sharp, nutty odor tells you trouble’s brewing. Lab rooms with underground ventilation perform better than those with small fans. My advice always goes: test the air, let the hoods run, and don’t work alone during big transfers. No chemical likes a crowded, stuffy space, and 1-Methylpyrrole hates it more than most.
Glass bottles with tight-fitting lids stopped a hundred headaches before they started. The chemical eats through low-grade plastics, softening them into a mushy mess. If you store 1-Methylpyrrole anywhere near acids or oxidizers, sparks could fly—literally. Good labeling saves the day, more than once, keeping surprise reactions at bay. For spills, I watched absorbent pads soaked with sand do wonders. The temptation to mop with paper towels or pour it down the drain bites back with plumbing bills and angry emails from safety teams. Collecting waste in dedicated containers, and logging every drop, sets up a safer workspace for the long haul.
No one walks into chemistry ready for everything, but building good habits early pays off. 1-Methylpyrrole’s safety sheet reads like a warning, but that’s for a reason. Folks who shrug off the instructions find themselves in trouble fast. Simple things—extra gloves in a drawer, fresh air in the room, solid waste labels—spare a world of pain. Establishing a routine means trouble becomes rare, not frequent. I’ve worked with teams who meet before every session, check equipment, swap stories about workplace scares, and learn from each one. The more stories shared, the better everyone handles the next tricky bottle.
Safe handling isn’t a checklist; it’s an attitude. Respect grows with every shift and every bottle poured safely back on the shelf. 1-Methylpyrrole makes for useful reactions and discoveries. It deserves careful treatment, and a crew ready to look out for each other.
Open any chemical supplier's website and you'll spot 1-Methylpyrrole in a number of purity grades: technical, reagent, and sometimes "extra pure." This isn’t laboratory trivia—it’s chemistry that spills over into everything from research labs to commercial syntheses and sometimes, trouble. Ask any researcher who’s tried getting a flaky reaction to work; purity of that bottle becomes the main suspect.
Different projects ask for different levels of trust in a chemical. Think of old-school paint manufacturing—technical grade usually fits the bill. Factories rarely need something 99.9% pure to make paint stick. On the other hand, pharmaceutical research gets strict about impurities. Nobody wants mystery by-products showing up in a drug’s test batch, so those teams pay the premium for higher purity. It takes one failed experiment due to a sneaky impurity to understand the risk.
Plenty of times, someone new to the bench will order the cheapest grade, thinking all 1-Methylpyrrole is the same. But the side-products and trace water in a low-grade bottle can sabotage a job, sometimes in subtle ways. Chromatography gets messy, yields suffer, by-products creep in. Even instruments can clog up. A chemist once told me his NMR machine signaled “no” with blobby baseline humps because the sample wasn't clean enough.
On the flip side, grabbing ultra-pure 1-Methylpyrrole when the project only asks for a bulk synthesis or surface coating wastes money. It’s the same as using gourmet olive oil to fry frozen fries—you don’t get the payoff.
Picking the right purity can mean chasing down Certificates of Analysis to match regulatory demands. When labs push for greener reactions or try to scale up, trace contaminants—metals, water, extra aromatic compounds—start to matter more. Forgetting to check for those factors can undo weeks of grind.
Some suppliers cut corners by not providing full data or selling “purified” stock that only looks good on paper. Anyone running a critical synthesis has to dig into product specifications or request extra documentation. Sometimes, it’s about calling the supplier and pressing for batch-specific details, even sending samples out for third-party testing.
People who order chemicals at scale know the drill: don’t skimp on requests. Be upfront about the intended use, and look for suppliers offering true quality control—not just slick brochures. Communicate with vendors who are transparent. Build up a trusted list. Employers sometimes miss this, but training researchers and procurement teams to recognize the practical risks of low-grade chemicals avoids costly reruns and lab disasters.
For researchers and small businesses, pooling orders or partnering with academic or industrial consortia makes higher purity affordable. In some cases, distilling or purifying in-house works, if the team really knows the method and can monitor purity with good instruments. It’s the old rule—test before you trust.
Working with 1-Methylpyrrole or any other fine chemical takes more than just a quick glance at the catalog grade. Real-world chemists and operators share stories about how a few percent difference in purity forced late-night fixes. Anyone serious about their product quality takes chemical sourcing seriously, and cuts out the guesswork wherever possible.
| Names | |
| Preferred IUPAC name | 1-Methyl-1H-pyrrole |
| Other names |
1-Methyl-1H-pyrrole N-Methylpyrrole N-Methyl-1H-pyrrole 1-Methylpyrrol N-Methylpyrrol |
| Pronunciation | /waɪˈmɛθɪlˌpɜːrˌoʊl/ |
| Identifiers | |
| CAS Number | 96-54-8 |
| Beilstein Reference | 1209246 |
| ChEBI | CHEBI:36657 |
| ChEMBL | CHEMBL48637 |
| ChemSpider | 13909 |
| DrugBank | DB04268 |
| ECHA InfoCard | 100.012.716 |
| EC Number | 01-2119971136-36-0000 |
| Gmelin Reference | 7927 |
| KEGG | C06178 |
| MeSH | D017708 |
| PubChem CID | 7923 |
| RTECS number | UY3150000 |
| UNII | 934E9R51KW |
| UN number | UN2305 |
| Properties | |
| Chemical formula | C5H7N |
| Molar mass | 81.12 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | amine-like |
| Density | 0.969 g/mL at 25 °C |
| Solubility in water | slightly soluble |
| log P | 0.85 |
| Vapor pressure | 3.1 mmHg (25 °C) |
| Acidity (pKa) | 17.0 |
| Basicity (pKb) | c. 13.1 |
| Magnetic susceptibility (χ) | -28.2 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.487 |
| Viscosity | 1.61 mPa·s (25 °C) |
| Dipole moment | 1.74 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 208.6 J∙mol⁻¹∙K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | 99.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2856.6 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Precautionary statements | H225,H315,H319,H335 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 38 °C |
| Autoignition temperature | 315 °C |
| Explosive limits | 1.8–11.4% |
| Lethal dose or concentration | LD₅₀ (oral, rat): 900 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 820 mg/kg |
| NIOSH | NIOSH: QD0525000 |
| REL (Recommended) | 0.07 |
| IDLH (Immediate danger) | 500 ppm |
| Related compounds | |
| Related compounds |
Pyrrole Pyrrolidine Pyrrolidone 2-Methylpyrrole 3-Methylpyrrole |