4-Chloro-1-Methylpiperidine didn’t just pop up out of a modern research lab. The story goes back to the early days of piperidine chemistry, a backbone in synthetic organic chemistry since the late 1800s. Workers found that playing around with piperidine derivatives could open doors to entirely new classes of pharmaceuticals. Adding a methyl group at the nitrogen atom but leaving a free 4-position had a tantalizing pull, and the introduction of a chlorine atom at this spot took the molecule in some new directions. By the 1960s, researchers started isolating 4-chloro variants for various alkaloid studies, and it wasn’t long before big chemical manufacturers entered the scene, refining production routes and sifting through this compound's unique features. Each generation of chemists—never just happy with what they already knew—kept pushing the boundaries, using it as a starting point or a key intermediate for new drugs, agrochemicals, and even polymers.
4-Chloro-1-Methylpiperidine belongs to the family of chlorinated piperidines. Its backbone comes from the six-membered piperidine ring, with a chlorine atom sitting at the fourth carbon and a methyl group stuck to the nitrogen. The real world calls for this molecule in fairly selective applications and not just academics. You’ll run into it across contract research labs, as a building block for more complex chemical architectures or as a key intermediate in synthesizing specialist drugs and pesticides. Availability depends largely on region, manufacturer, and local demand—but it is not usually a shelf product outside of high-end lab suppliers. You often run into it as an off-white crystalline solid, sealed tight from air and moisture, often packed in containers ranging from tiny gram bottles for research use up to larger cans for industrial needs.
If you put a sample of 4-Chloro-1-Methylpiperidine on your lab bench, you’d notice its faint crystalline appearance pretty quickly. Usually, it clocks in with a melting point below 50°C, which means it liquifies on a warm day. Its boiling point floats above 210°C, suggesting reasonable thermal stability, which helps manufacturers survive purification runs without losing too much product. The molecule is moderately soluble in most organic solvents—ethers, acetone, and even chloroform, but don’t expect to stir it into water without trouble. Its structural skeleton tends to make it reasonably stable in the bottle, though high humidity or a careless storage spot might lead to slow hydrolysis or decomposition over time. The chlorine atom opens up the molecule to nucleophilic substitution reactions, making it a handy entry point for more complex syntheses. If you take a deep breath around it, you’ll quickly remember the sharp, amine-like odor that lingers with many nitrogen heterocycles—definitely not a compound to waft for fun.
From the supplier’s side, the product’s purity stands as the critical detail. Common practice involves reporting purity by GC or HPLC, usually notching above 95% for research-grade material. Labels ought to show CAS number 934-25-8, full chemical name, and a batch number for traceability. SDS paperwork typically flags the molecule as a hazardous substance, due to its toxicity and potential health impacts, with clear guidance on protective gear and storage requirements. Because this compound can have shelf stability issues if not capped or kept away from moisture and strong light, expiry dates or retest periods are often included on the label. The technical dossier usually mentions molecular weight (133.61 g/mol), empirical formula (C6H12ClN), and IR or NMR data for identity checks. Suppliers typically offer recommendations for safe handling, packaging configurations, and proper waste disposal, since mishandling or accidental spillage poses risks both to individual health and the environment.
The most common route to 4-Chloro-1-Methylpiperidine uses either direct chlorination of 1-methylpiperidine or, more selectively, ring formation from N-methyl-4-chlorobutanamine under suitable conditions. Direct chlorination tends to yield a mixture of isomers, and chemists often use protecting groups or directed chlorination agents to funnel the reaction to the fourth position on the ring. After reaction, purification by distillation or crystallization cleans up the bulk of by-products. The industry also leans toward patent-protected methods, combining high yields with controlled waste streams, and some specialized plants may use solid-phase synthesis if batch size or purity calls for it. Either way, it takes a skilled hand to avoid the side reactions that can easily chew through valuable starting material.
Manipulating 4-Chloro-1-Methylpiperidine’s structure can take a chemist in dozens of directions. The chlorine atom acts like a red flag for nucleophilic attack, so you can swap it out for all kinds of groups: amines, thiols, or carboxylates, each leading to new piperidine derivatives with distinct characteristics. Laboratories eager for new pharmacophores will use this compound as a precursor, often carrying out alkylation or acylation at the nitrogen atom to decorate the molecule for drug discovery. The core ring is robust, but under tough conditions, ring opening can create linear molecules for agrochemicals. Oxidation at the methyl site, or reductive dechlorination, can further expand the catalog of products available from this intermediate. Its versatility creates a playground for creative synthetic planning, and it serves as a springboard for both minor modifications and major transformations.
Chemical suppliers and research catalogs list plenty of synonyms for 4-Chloro-1-Methylpiperidine. Some catalogs call it N-Methyl-4-chloropiperidine, or just Methyl-4-chloropiperidine. Look hard enough, and you’ll spot it under CAS 934-25-8. In older literature, you might see the names played with slightly— like 4-chloro-N-methylpiperidine. Shorthand often simplifies to 4-Cl-N-Me-pip among synthetic chemists, especially in the notes column of lab notebooks or in online procurement systems. This helps to avoid confusion, especially in settings where dozens of piperidine derivatives float around.
Safety dominates any conversation about handling 4-Chloro-1-Methylpiperidine. Prolonged skin or eye contact spells trouble, thanks to corrosive and toxic effects that can show up fast. Lab workers must gear up with gloves, goggles, and protective coats. Fume hoods aren’t just recommended—they’re essential, because vapor inhalation irritates the respiratory tract and, in high doses, can lead to systemic poisoning. Facilities storing this compound set strict temperature and ventilation controls, and all containers carry secondary containment to catch leaks or accidental drops. Spill protocols emphasize immediate cleanup with absorbent material, strict avoidance of water, and proper disposal of contaminated gear through licensed waste handlers. Legal standards in many countries put this molecule on lists for hazardous chemicals, regulating transport, storage, and use through safety data sheets and periodic audits.
Most end users treat 4-Chloro-1-Methylpiperidine as a synthetic stepping stone. It shows up heavily in pharmaceutical R&D, where teams chase new treatments for pain, neurological disorders, or metabolic disease. Medicinal chemists covet this backbone for modifying lead compounds, tuning efficacy and shifting metabolic stability. Agrochemical firms see value in the molecule for prepping selective herbicides or pesticide scaffolds, looking to tap into the peculiar reactivity it offers. There’s also some attention in developing specialty polymers and materials, especially where nitrogen heterocycles boost performance. Any chemist who needs to swap functional groups onto a heterocyclic ring, and who wants a straightforward handle for further construction, tends to keep a stash on the shelf.
Innovation in synthetic chemistry continues to pull 4-Chloro-1-Methylpiperidine into new projects. Drug discovery often nudges structural tweaks for better target selectivity or reduced side effects. Teams seeking to develop greener synthesis push for solvent-free methods, improved atom economy, and more benign reagents—promising lower costs and safer products. Collaboration across university labs and industry groups has also thrown light on using this compound in the assembly of supramolecular structures, catalysts, or even sensors. As more high-throughput synthesis equipment comes online, it finds a spot in automated chemical platforms, studied in thousands of reactions and cross-checked by AI tools searching for new routes to high-value targets. My own time in a medicinal chemistry group taught me the importance of robust intermediates—we relied on molecules like this to probe hypotheses, optimize yield, and, sometimes, rescue projects from dead ends.
Researchers have logged case studies and animal data, flagging 4-Chloro-1-Methylpiperidine as a substance that shouldn’t be underestimated. Acute toxicity studies note that even small oral or vapor exposures trigger pronounced CNS symptoms and possible liver effects. Chronic exposure ramps up the risk for cumulative toxicity—not just at the molecular level, but system-wide effects. Environmental fate studies remain limited, but the chlorine atom hints at possible persistence and bioaccumulation risks. Standard practice calls for containment, minimal exposure, and engineering controls, because spills can produce hazardous byproducts, and uncapped vials degrade air quality in shared lab spaces. No one with real lab experience will shrug off the warnings on the safety sheet or ignore the need for regular health checks.
The world of specialty chemicals and drug discovery keeps shifting, and 4-Chloro-1-Methylpiperidine sits on the edge of this movement. Sustainable synthesis will probably drive new preparation routes, as chemists look for efficiency and reduced impact. Regulatory pressures might tighten around toxic intermediates, nudging manufacturers to rethink batch size and packaging. Market demand could tilt as newer piperidine analogs claim the stage, but for now, this compound remains a sturdy pillar where unique reactivity and established performance mean something. Ongoing research might finally fill in gaps around its biodegradation and chronic toxicity, supporting safer protocols and better environmental stewardship. The road ahead demands vigilance, skill, and a respect for the limits of chemistry—traits any seasoned hand in the lab knows matter as much as molecular structure itself.
4-Chloro-1-Methylpiperidine pops up in chemistry labs and pharmaceutical plants as a key intermediate. Scroll through chemical supplier catalogs, and purity levels jump out: 97%, 98%, even the rarer 99%. Each bump in those numbers means a different price tag and a different audience. The academic world and research outfits look for that 97-98% mark, feeling comfortable with some minor impurities if it means saving a little. Large pharmaceutical companies, making sensitive or high-value compounds, reach toward the cleaner, 99% version.
Back in my own research days, I watched what a low-purity sample does to a complicated reaction. Sometimes a few percent of unidentified byproducts throws off a whole synthesis, turning hours of work into confusion. Other times, that same sample gets the job done in a classroom demo that doesn’t call for surgical precision. The real clue about what grade a buyer should choose comes from what rides along with the piperidine—residual starting materials, solvents, and trace water. Impurities sometimes slip by unless analytical checks happen before the bottle hits the shelf.
Bulk buyers push for high reproducibility and clear certificates of analysis. They want to see numbers on every batch: HPLC, GC-MS, and NMR, not just a sticker that says “98%”. Smaller operations or academic labs often get by with fewer guarantees if costs run high. Buying from established suppliers like Sigma-Aldrich, Alfa Aesar, TCI, or Oakwood, high-purity grades hit the market with certified documentation, but these perks rarely come cheap. I’ve watched startups try to trim purchase lists, swapping a 98% reagent for a 97% version. Sometimes that gamble pays off. Sometimes a reaction batch fails, forcing a redo with cleaner ingredients.
People sometimes underestimate how quickly unwanted chemicals pile up in repeated use. Trace halides, amines, or water and those above 2%—they will show up if the end use isn’t forgiving, like drug synthesis or making reference standards. The folks chasing lower prices from less-known suppliers sometimes run into shelf-life problems, inconsistent analysis, or skewed labeling. Product recalls happen, losing days and dollars nobody budgeted for. Trust builds over time. Once suppliers show steady data for their products, chemists stick with them, knowing that the purity won’t dip on the next purchase.
As the demand for 4-Chloro-1-Methylpiperidine rises, especially in custom synthesis and specialized research, suppliers face pressure to be up front with their documentation. Industry watchdogs and academic journals are asking for chromatograms over guesswork. Online reviews help; research teams share results—good and bad—about what’s really in the bottle. Digital transparency bridges the trust gap. If more companies followed through with QR code certificates, real-time batch tracking, and shared analytical data, everyone stands to win. Meanwhile, chemists gain by double-checking every delivery, even from their trusted brands. One batch of suspect intermediate ruins more than a reaction—it wastes funds and time that nobody gets back.
Anyone who’s handled chemicals in a lab knows the difference between a clean, worry-free bottle and a disaster waiting to happen. 4-Chloro-1-Methylpiperidine stands out for its utility in synthesis, but that comes with strings attached. This stuff won’t forgive careless mistakes. The slightest error can turn a straightforward day of mixing into a safety briefing nobody asked for.
Stick this compound in a warm spot—say, next to a sunny window or above a humming radiator—and you’ll smell trouble before you see it. Heat can stir up volatility, lifting fumes into the air. Take it from anyone who’s cracked open a bottle after neglecting temperature rules. The air stings, and your lab mates make it clear—don’t do that again.
I never forget the time someone ignored the low and steady approach. Proper storage means seeking out a spot with cool air, not freezing but always consistent. Avoid temperature swings. Fluctuations mess with purity and can push pressure up in sealed containers. Room temperature, closer to the lower end, somewhere around 15 to 25 degrees Celsius, works best.
Humidity loves causing trouble with chemicals. Moisture in the air can sneak into bottles, changing physical properties, sometimes with nasty surprises. Keep storage spaces dry, away from any possible leaks, and steer clear of high-traffic water sources.
Direct sunlight poses another hidden threat. Rays break down bonds in organic compounds, sometimes faster than you’d guess. Coverage matters—opaque or amber-glass bottles serve a purpose. I know colleagues who saved themselves big headaches by using the right containers, stashing bottles away from light-heavy shelves.
Labels tell everyone what’s inside. In a shared space, you can’t rely on memory alone. One slip means someone might reach for the wrong bottle, and in chemistry, that mistake carries real consequences. Whenever I set up my shelves, I take the time to write clear, bold labels, checking them every few months for readability.
Sealing bottles well keeps fumes in and moisture out. A leaky cap or half-closed jar lets vapors escape, and you’ll smell the difference. Good seals also lower the risk when moving bottles from one bench to another.
Storing 4-Chloro-1-Methylpiperidine next to acids, oxidizers, or flammable solvents creates unnecessary risks. This isn’t just red tape—it’s how real-world accidents happen. I once saw what happens after a mislabeled solvent leaks. The residue didn’t take long to pick up, but the cross-contamination meant writing off most of a year’s supply. Segregation matters as much as any other precaution.
Investing in ventilated, lockable storage pays off long-term. Good air flow means fewer headaches and less chance of something lingering in the lab air. Routine checks on expiration and container integrity keep surprises at bay. Training new team members on these points saves everyone time and lots of stress.
Chemical storage won’t ever be glamorous, but with the right approach—one that respects the properties of each compound—you rest easier at the end of the day.
4-Chloro-1-methylpiperidine isn’t something you find in your kitchen cabinet or under your bathroom sink. It pops up mainly in research labs and chemical plants, playing an undercover but important role in the world of organic synthesis. This molecule draws attention because of its structure—a piperidine ring with a chlorine atom and a methyl group attached. For chemists, that small shift in structure can change everything.
In pharmaceutical circles, people care a lot about piperidine rings. They’re common in many drugs, and swapping around atoms on that ring can unlock new possibilities. With 4-Chloro-1-methylpiperidine, research teams can build more complex molecules by swapping out the chlorine for other groups. This trick helps them test new candidates for painkillers, antidepressants, or antivirals. If you comb through drug patents, you’ll spot plenty of piperidine cousins, including those made with this very building block. Often, these tweaks can mean the difference between a medicine that works and one that sits forgotten on the shelf.
Big farms don’t function without solid chemistry behind the scenes. Companies developing crop protection chemicals—herbicides, pesticides, fungicides—rely on good starting materials. 4-Chloro-1-methylpiperidine shows up in test batches for new pesticides. Its unique combination of chlorine and methyl gives companies the option to dial-in features that control how a chemical breaks down, how safe it is, and how it moves inside a plant. A smart tweak at this stage can make a spray stick to leaves longer or help it break apart quickly after doing its job.
Beyond medicines and pesticides, this molecule brings order to the chaos of organic chemistry. Some chemical reactions seem unruly—side-products form, or the target molecule takes ages to appear. Chemists sometimes use 4-Chloro-1-methylpiperidine as an intermediate or a helper in these cases. As a nucleophile or a base, it can steer tricky reactions the right way. More efficient or cleaner results can save a lab valuable time and stretch research budgets.
While not a staple in mainstream industry, this piperidine finds its way into specialty materials. Certain polymers and coatings demand precise molecular tweaks. With its chlorine and methyl hook-ups, 4-Chloro-1-methylpiperidine allows for interesting cross-linking or branching during polymer preparation, especially in niche electronics or high-performance adhesives.
One look at the hazard label and it’s clear: handling this chemical brings certain risks—skin, eye, and respiratory irritation all show up as warnings. Labs that use it have to be strict about safety gear and air filters. While its usefulness can’t be dismissed, there’s always room for improved handling protocols and greener reaction conditions. One possibility involves tweaking the synthesis to be less wasteful. Another approach comes from the world of green chemistry—reduce the need for hazardous solvents or switch out the chlorine atom for something less toxic. These aren’t simple fixes, but momentum in the chemical industry keeps pushing for safer, more sustainable alternatives.
Anyone who’s spent time in a lab knows the importance of trusting what’s in the bottle. Walking down the aisles of a chemical storeroom, you get pretty good at spotting the basics — date received, manufacturer, hazard symbols. But, in this industry, nobody takes anything at face value. Enter the Certificate of Analysis, or COA, which is more than just a formality. It’s the document that proves what the label says. For a niche compound like 4-Chloro-1-Methylpiperidine, this becomes even more important.
A COA isn’t just a checklist. It’s a snapshot of how that specific batch measures up, listing out everything from identification methods to purity percentages and data on trace impurities. Just last year, I worked on a project that stumbled because the amine we ordered came in a little off-spec. Even a half-percent unknown can mess with your reactions. These surprises don’t just delay progress; they eat into budgets and raise safety questions. If your supplier won’t share a COA for something as specialized as 4-Chloro-1-Methylpiperidine, you find yourself rolling the dice every time you place an order.
4-Chloro-1-Methylpiperidine isn’t something you pick up from a corner store. Its uses can show up in pharma, agrochem, or research settings, and buyers aren’t just hobbyists – many are professionals with deadlines and strict protocols. In an academic setting, a failed reaction can cost valuable time. In industry, regulatory concerns stack up fast. Without proof of substance purity, every downstream application is open to risk. We’re talking about reactions not running as planned, regulatory fines, or worst-case, putting unsafe batches on the market. Manufacturing cycles run on tight schedules, and a questionable batch can lead to a full shutdown.
Finding a reliable COA for rare chemicals often boils down to the supply chain. Some suppliers treat documentation as optional, especially for low-volume chemicals. Smaller firms might not have the quality control measures that bigger players adopt by default. I once spent three weeks negotiating with an overseas vendor for COA data, only to receive a generic PDF unrelated to our actual shipment. Repeat that headache across a busy research group, and you’ll see why this isn’t just bureaucracy — it’s about making research and manufacturing safer and more predictable.
Anyone buying 4-Chloro-1-Methylpiperidine should treat the COA as a non-negotiable. If a supplier hesitates or says documentation will come after delivery, that’s a red flag. Folks can check for suppliers who hold ISO certifications or similar third-party audits. It’s worth digging into a company’s track record rather than just taking a sales pitch at face value. At the buyer’s end, setting up a purchase policy that demands batch-specific COAs before payment adds leverage. Labs can back this up with their own in-house checks to spot anomalies early. A little vigilance on documentation pays dividends, cutting down on risk and wasted money.
Ordering chemicals like 4-Chloro-1-Methylpiperidine isn’t as direct as picking up flour or sugar at a grocery store. Suppliers set minimum order quantities (MOQ), often between 1 to 25 kilograms, not to frustrate buyers, but to balance lab safety, production costs, and international shipping issues. Smaller producers—startups, research labs, even veteran chemists in new fields—often run into these hurdles at the worst moments. There’s an ocean between a small synthetic experiment and a full-on industrial effort, but the gatekeeper always seems to be MOQ.
Ordering just what you need looks logical on paper. After all, why stock extra kilos of a specialty compound that you may not use for years? Safety rules for hazardous chemicals pile on complexity. Smaller vials cost more per gram. Bulk orders—say, 5 kg or more—push storage rules, regulatory filings, and budget caps. For a while, I learned this the hard way: facing down a supplier who wouldn’t break a case, knowing my project needed less than 50 grams.
Every supplier manages production lines, labor, waste, and packaging. Running a synthesis isn’t cheap, and storing a half-ton drum for months burns warehouse space fast. Regulatory paperwork, especially for controlled or flagged chemicals, adds another layer of cost. International shipping runs into dangerous goods declarations, paperwork for customs, and specialized containers—no one wants that for a single flask’s worth.
For 4-Chloro-1-Methylpiperidine, these issues stack up. The compound finds its way into pharmaceutical research and new material testing, which brings high scrutiny and extra paperwork. Producers often lump small orders into “pilot batches,” letting a dozen buyers split the effort, but this requires coordination and trust. Some sellers, concerned about end-use and diversion, only sell to established labs—each jump in order size reassures them someone is serious.
Small labs often wind up begging colleagues for surplus, or turning to gray-market suppliers—risky, with headaches ranging from uncertain purity to real legal jeopardy. Some university consortia or research networks solve the MOQ roadblock by pooling orders; a handful of institutions combine demand, then divvy up shipments at their own storage sites. This method saves cost and reduces the challenge of hazardous handling, since shipping fewer, larger packages means fewer opportunities for loss or accident.
Another solution: be transparent. Reaching out to suppliers, explaining the research need, and showing compliance with storage and safety rules has helped me unlock access to “off spec” or short-dated lots in the past. Factories sometimes have remnant stocks, leftovers from a production run too small for their regular clients, but too large for a single lab. Patience and negotiation open more doors than most realize.
No single fix will erase the challenge of high MOQs for specialty chemicals such as 4-Chloro-1-Methylpiperidine. But talking openly with suppliers, sharing goals with peer labs, and staying alert for programmatic group buys can break the problem into manageable pieces. For anyone frustrated by the system, remember: the weird math behind MOQs follows real-world costs, not just arbitrary rules. Getting ahead means mastering the network as much as the chemistry.
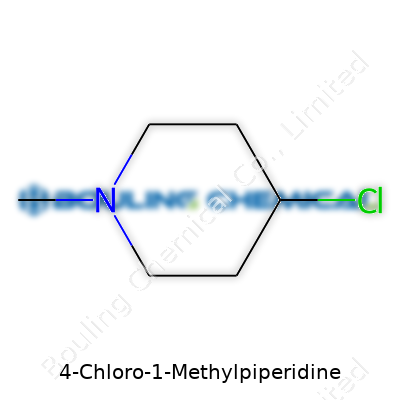
| Names | |
| Preferred IUPAC name | 1-methyl-4-chloropiperidine |
| Other names |
4-Chloro-1-methyl-piperidine N-Methyl-4-chloropiperidine 1-Methyl-4-chloropiperidine 4-Chlor-1-methylpiperidin 4-Chloro-N-methylpiperidine |
| Pronunciation | /ˈklɔːroʊ waɪ ˈmɛθɪl paɪˈpɪrɪdiːn/ |
| Identifiers | |
| CAS Number | 104897-67-6 |
| 3D model (JSmol) | `/3Dmol%20viewers/JSmol.htm?model=CC1CCN(CC1)Cl` |
| Beilstein Reference | 1720563 |
| ChEBI | CHEBI:189669 |
| ChEMBL | CHEMBL489772 |
| ChemSpider | 21634368 |
| DrugBank | DB14658 |
| ECHA InfoCard | 03c5985a-1382-4c76-89b7-2f5cc1c16d53 |
| Gmelin Reference | 84157 |
| KEGG | C19274 |
| MeSH | D017962 |
| PubChem CID | 12518967 |
| RTECS number | TM3674000 |
| UNII | 4HT9V7QL0G |
| UN number | UN3431 |
| Properties | |
| Chemical formula | C6H12ClN |
| Molar mass | 133.62 g/mol |
| Appearance | Colorless to light yellow liquid |
| Odor | Amine-like |
| Density | 0.99 g/mL at 25 °C (lit.) |
| Solubility in water | Slightly soluble |
| log P | 1.82 |
| Vapor pressure | 0.4 mmHg (25°C) |
| Acidity (pKa) | pKa = 11.2 |
| Basicity (pKb) | 3.43 |
| Magnetic susceptibility (χ) | -67.4×10⁻⁶ cm³/mol |
| Refractive index (nD) | n^20_D^ 1.487 |
| Viscosity | Viscous liquid |
| Dipole moment | 3.61 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 325.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -83.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4558.7 kJ/mol |
| Pharmacology | |
| ATC code | |
| Hazards | |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Danger |
| Hazard statements | H302, H314 |
| Precautionary statements | P210, P261, P280, P301+P312, P303+P361+P353, P305+P351+P338, P370+P378 |
| NFPA 704 (fire diamond) | 1-3-0 |
| Flash point | Flash point: 63 °C |
| Autoignition temperature | 270 °C |
| LD50 (median dose) | LD50 (median dose) = 290 mg/kg (oral, rat) |
| NIOSH | UK1560000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.5 ppm (2 mg/m³) |
| IDLH (Immediate danger) | NIOSH has not established an IDLH value for 4-Chloro-1-Methylpiperidine. |
| Related compounds | |
| Related compounds |
1-Methylpiperidine 4-Chloropiperidine 4-Fluoro-1-methylpiperidine 4-Bromo-1-methylpiperidine 4-Chloro-1-ethylpiperidine 1,4-Dimethylpiperidine 1-Methyl-4-nitropiperidine 1-Methyl-4-hydroxypiperidine |