Chemistry has left its mark on every era, and the story of 1-Methylpiperidine-2-ethanol fits the pattern. In the postwar decades, demand for alkaloid analogs and specialty amines grew as researchers looked for molecules with unique pharmacological profiles and synthetic flexibility. During the mid-twentieth century, improvements in catalytic hydrogenation and selective alkylation put such heterocyclic amines within reach. Laboratories moved beyond basic piperidine, exploring methyl substitution and alcohol side chains. As custom syntheses increased, chemical suppliers took notice and started listing these materials, especially as drug screening programs and agrochemical innovation required more structure–activity exploration. The journey of 1-Methylpiperidine-2-ethanol rides alongside the practical need for functionalized amines and the wider expansion of medicinal chemistry toolboxes.
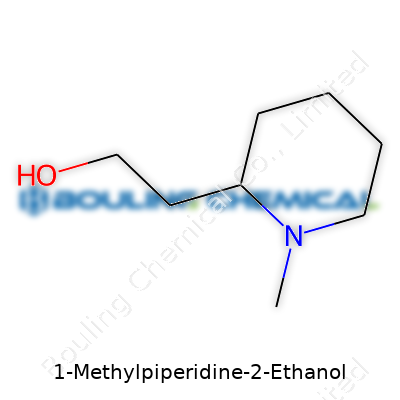
1-Methylpiperidine-2-ethanol shows up in chemical catalogs as an off-white to pale yellow liquid or low-melting solid. Chemists recognize its structure at once: a six-membered nitrogen ring, with a methyl group at the first position and a hydroxyethyl side chain on the second carbon. It balances water-solubility from the ethanol group and organic solubility from the saturated ring. Lab teams interested in piperidine derivatives pull this compound for its synthetic potential—ready to undergo further reactions at the alcohol, the secondary amine, or along the backbone. This structure appeals to researchers interested in making analogs for biologically relevant tests or for building block purposes in organic synthesis. Bottle labels often list typical specs above 98% purity for research grades, satisfying the accuracy needed for analytical and developmental work.
This small molecule packs physical traits that make it easy to handle in most laboratory settings. Its molecular formula—C8H17NO—gives a molar mass around 143.23 g/mol. Its low melting point keeps it liquid or semi-solid under standard room conditions, which makes weighing samples simple and straightforward. Like other secondary amines, 1-Methylpiperidine-2-ethanol carries a faint nitrogenous odor—noticeable, though much less pungent than unadorned piperidine. The hydroxyl group boosts its hydrophilicity, so users find that the compound dissolves in water and polar organic solvents. Its boiling point comes high enough to allow distillation under reduced pressure without significant decomposition, setting it up for purification by vacuum distillation or short-path setups. The molecular architecture makes both the nitrogen and oxygen sites accessible, useful for downstream chemical elaboration.
Chemical suppliers print specifications out of necessity. Most bottles of 1-Methylpiperidine-2-ethanol come with analytical results—liquid chromatography, NMR spectra, and sometimes elemental analysis for reassurance. The label usually lists storage recommendations: keep cool, protect from moisture and strong oxidizers. Some stockrooms request refrigeration to prevent any slow oxidation or degradation. Most research-grade products require a purity of no less than 98%, with water and residual solvent levels below 0.5%. SDS sheets spell out the flash point, boiling range, irritancy, and incompatibilities, so everyone along the chain—from bench chemist to safety officer—knows what to expect.
Synthesis of 1-Methylpiperidine-2-ethanol calls for a blend of ring manipulation and chain-building. The piperidine ring takes shape through classic methods—cyclization of aminopentanes or reductive amination routes. Methylation can happen before or after ring closure, but direct N-methylation with agents like methyl iodide or methyl sulfate on piperidine is popular for efficiency. Adding the hydroxyethyl group often uses alkylation: reacting the methylpiperidine with an ethylene oxide or halohydrin under basic conditions leads to the hydroxyethyl group linking onto the ring. Careful control of temperature and time minimizes side products, such as dialkylation or over-alkylation at the nitrogen. Work-up steps remove inorganic salts and unreacted reagents, and the final distillation cleans up the end product.
The structure of 1-Methylpiperidine-2-ethanol opens routes for chemists who want to push boundaries. The secondary alcohol on the ethyl chain can become a reactive site—oxidation transforms it into a ketone, or esterification links it with carboxylic acids for more elaborate structures. The N-methylpiperidine ring often acts as both nucleophile and base, joining in with alkyl halide substitution, condensation with activated carbonyls, or deprotonation to form imines. Reductive amination, carbamate formation, and sulfonylation at the nitrogen also appear in the literature. Med chem teams tune substitution patterns along the ring and at side chains to probe biological activity or develop SAR maps for CNS-active or antimicrobial compounds. Further, derivatizing the ethanol moiety builds potential prodrugs or increases lipophilicity—a trick often used to test structure–function relationships.
Suppliers sometimes differ in how they name 1-Methylpiperidine-2-ethanol—variations include 2-(1-Methylpiperidin-2-yl)ethanol, N-Methyl-2-piperidinoethanol, or Methylpiperidinoethanol. Catalogs sometimes group it with similar piperidinol derivatives, so searching by CAS number avoids mistakes. Researchers often refer to it in shorthand—“NMP2E” appears in lab books—but nomenclature best practice keeps to systematic names. This consistency matters, especially as the compound moves between chemical supply chains and regulatory bodies around the world.
Like many small amines, 1-Methylpiperidine-2-ethanol earns its safety data sheet. Direct exposure may irritate the skin, eyes, or respiratory tract, so gloves, goggles, and adequate ventilation rank as standard lab precautions. Its flash point sets it firmly outside the highly flammable category, but good chemical sense keeps it away from hot plates or open flames. Reactivity concerns tie back to strong oxidizers, acids, and alkylating agents—common for amines but always worth remembering. Waste streams need collection in organic waste bins, and emergency protocols ask for soap, water, and plenty of airflow in the event of a spill. Long experience shows that slow, deliberate handling reduces incidents, a lesson many newcomers learn on their first day in the synthetic lab.
1-Methylpiperidine-2-ethanol serves as a versatile intermediate across pharmaceutical and agrochemical development. Drug discovery programs value the piperidine core for its CNS penetration, metabolic stability, and receptor binding properties. The molecule also fits screening runs for anticholinergic, analgesic, and antihistamine activity. Agrochemical researchers use the structure for novel pesticide leads and plant-growth-modifying compounds. Because it contains both nucleophilic nitrogen and reactive alcohol, 1-Methylpiperidine-2-ethanol often launches as a starting building block when diversity in a chemical library matters. Educators draw on it for advanced organic laboratory classes, where students practice SN2 alkylations, amination, and straightforward oxidations. Manufacturers experiment with derivatizing the piperidine ring for flavor or fragrance additives, though regulatory concerns slow such experimentation.
Chemical innovation depends on these “middleweight” molecules, where tuneable groups unlock new findings. Research on 1-Methylpiperidine-2-ethanol runs from basic synthetic methodology to pharmacological evaluation in cell or animal models. Teams use it to test how ring nitrogen substitution changes metabolic fate or molecular recognition. Publications cited in SciFinder or PubChem reveal a network of synthetic schemes, either making the molecule in fewer steps or using it as a node for more complex architectures. Sometimes the target molecule falls outside pharmaceuticals—polymer or surface science labs see potential in using this amine as a modifier to change surface properties or improve adhesion in specialty coatings. These efforts reflect larger patterns in chemistry, where old scaffold molecules can suddenly become valuable once a new use or reaction comes to light.
Toxicity studies for 1-Methylpiperidine-2-ethanol remain limited but informative. Standard cytotoxicity screening in mammalian cell lines points to low-to-moderate toxicity at practical concentrations, with dose-dependent effects on cell viability at higher exposures. Animal data—though still sparse—suggest that acute effects mirror those of simpler secondary amines, including CNS depression at high doses and reversible irritation after skin or eye contact. Chronic exposure studies have not appeared widely in the literature, but the presence of the piperidine ring and primary alcohol warn of metabolic liabilities once the molecule enters the body. Metabolic pathways likely involve N-oxidation, alcohol oxidation, and conjugation, so toxicologists recommend evaluating both parent compound and metabolites in any preclinical safety analysis. The lessons from related piperidine drugs and solvents advise a cautious approach in scaling up production or moving toward therapeutic application. Recent advances in in vitro predictive toxicology may speed the development of more precise hazard profiles.
Progress in heterocycle chemistry hints at a wider future for 1-Methylpiperidine-2-ethanol. Medicinal chemists continue to find interest in the piperidine motif, driven by the need for new CNS-active compounds and as a backbone for targeted ligands. As more screening platforms rely on combinatorial chemistry, easily modifiable building blocks like this ethanol-terminated amine stand to see greater demand. Green chemistry trends point toward new catalysts or biotransformations for the more sustainable synthesis of piperidine derivatives, which could bring 1-Methylpiperidine-2-ethanol into high-volume processes without the same environmental footprint as legacy methods. Academic-industrial collaboration already looks into non-pharmaceutical uses, such as polymer modification, advanced material design, or as templates for molecular recognition in sensor development. While regulatory concerns around secondary amines call for responsible stewardship and research, the breadth of potential applications ensures this compound remains part of the synthetic chemist’s toolkit for the foreseeable future.
Researchers and chemists often talk about 1-Methylpiperidine-2-Ethanol in the same breath as other versatile building blocks. Picture a workbench covered in tools and technical manuals; 1-Methylpiperidine-2-Ethanol is one of those tools you’d reach for when building new molecules—especially in pharmaceutical labs. The piperidine part isn’t just a chemical tongue-twister. It’s a structure you’ll find in countless medicines and experimental drugs. There aren’t a lot of everyday uses that pop up in the grocery store aisle, but the backbone of this compound matters to those aiming at medical breakthroughs or working on agrochemical projects.
Medicinal chemistry relies on compounds that offer flexibility during synthesis. A molecule like this ethanol derivative serves as a starting point to build more complex drugs. Chemists looking at new treatments for neurological or immune disorders often use piperidine-based scaffolds. Imagine designing a puzzle where several pieces can snap into different spots—this chemical makes snapping together new molecules much less of a headache.
I’ve seen firsthand how innovations in starting materials speed up the hunt for effective treatments. Instead of tweaking raw chemicals from scratch, labs pick proven frameworks like these to jump a few steps ahead. The idea is to reduce risk and avoid wasted time. For the pharmaceutical world, that means the difference between a two-year sprint and a four-year drag.
Crop scientists have their own stories with this type of compound. The structure lets people design and test safer alternatives for traditional pesticides, which need updating after decades of heavy use. Not everything in the chemical warehouse shifts straight to an application, but if researchers want to improve crop protection without harming pollinators or the environment, molecules in the piperidine family often come up in brainstorming sessions.
Industrial chemists look for stable, easy-to-handle chemicals for new coatings or additives. If a compound can serve as an intermediate to build better dyes, resins, or specialty plastics, plenty of attention follows. The sheer versatility of the piperidine “ring” structure, especially with added functional groups like the ethanol end, keeps it in the toolbox for those jobs.
Getting these chemicals in the highest quality matters. Even trace contaminants can mess up an experiment or slow down drug development. Labs rely on tight regulatory controls to make sure nothing slips in by mistake. Quality control costs money and adds delays, which then get passed on to the price tag of research projects or the final medicines.
Safe production is another huge piece of the story. Piperidine derivatives can be flammable or toxic in careless hands. Workers need solid training and careful handling procedures. Companies following best practices sign up for regular audits and documentation.
Supply chain transparency and regular third-party testing cut down on risk, both in quality and in safety. Some firms now use blockchain systems to track shipments and verify each batch by lot number. Bringing in artificial intelligence to flag irregularities or predict process issues has helped minimize costly recalls. More suppliers using these measures would uplift trust and speed up progress across industries.
Looking to the future, continued partnerships between manufacturers and researchers can drive down costs and raise standards. Opening communication channels about intended uses, potential hazards, and best practices means less guesswork and stronger outcomes for medicines and other essential products.
Working with chemicals like 1-Methylpiperidine-2-Ethanol isn’t just a technical challenge—it’s a daily decision about safety. This compound, with its distinct piperidine ring and ethanol tail, finds real-world use in labs and some industrial settings. Direct contact with skin or eyes could cause irritation. Breathing its vapors for too long sometimes brings dizziness or headaches. Glove selection matters—nitrile or neoprene give more protection than plain latex. Splash-proof goggles keep accidental sprays from leaving a mark.
Lab coats never seem enough without proper PPE. Every researcher I know insists on gloves, goggles, and full sleeves before pouring or mixing this compound. Even a small spill can soak into your clothes, so covering up helps. Chemical fume hoods are essential; no one should breathe these fumes directly. Spills remind us—sometimes loudly—that a little preparation stops big problems.
Storing 1-Methylpiperidine-2-Ethanol calls for cool, well-ventilated spots away from strong oxidizing agents. Tight, labeled containers make all the difference. At my last shared workspace, someone left a lid loose; a faint, sharp smell told us faster than any memo. Using secondary containment, like a plastic tray, prevents leaks and drips from spreading. Label everything clearly and never store with acids or bases that could react.
Spill kits don’t always get the love they deserve—until there’s a mess. Absorbent pads, neutralizers, gloves, and a well-practiced routine matter more than expensive gear. If this compound hits the skin, wash it off fast with soap and water. For eyes, nothing works quite like an eyewash station. On the rare chance someone inhales too much, fresh air is the top priority. Emergency numbers belong next to every fume hood, not in a dusty drawer across the hall.
Respect for the compounds you work with finishes at waste disposal. Pouring leftovers down the sink not only breaks regulations, but also harms water systems that everyone relies on. Use designated solvent containers. Labs that do it right have dedicated, dated logs and trained staff verifying what goes out. EPA rules might sound dry, but costly cleanups lurk behind every neglected bottle.
Safety talks aren’t a checkbox. Sharing firsthand stories—like the time someone learned the hard way about mislabeled bottles—sticks with new hires more than dry regulations. Making safety a team effort keeps everyone alert. Leaders who model good habits quietly set the tone. Small reminders, like double-checking lids or wiping down surfaces, build a culture where nobody takes shortcuts.
Protecting ourselves and our colleagues isn’t just about avoiding fines. It’s about getting home healthy every day. Products like 1-Methylpiperidine-2-Ethanol serve us best when we respect their potential. Personal vigilance, reliable equipment, and honest communication transform everyday hazards into manageable risks. In the lab, these steps go a lot further than any warning label.
Chemists know that every compound tells a story through its atoms and connections. 1-Methylpiperidine-2-ethanol shows this with its formula: C8H17NO. The backbone here is a six-membered ring called piperidine, where nitrogen takes the number one spot in the ring. A methyl group attaches to this nitrogen, giving the molecule extra stability and a punch in reactivity. Moving over to the carbon in the two-position, an ethanol side chain hangs off, defined by that -CH2CH2OH group.
For folks who like to picture molecules, think of a cyclic amine with a small alcohol tail. That tail opens up possibilities for interactions with other chemicals, making it more than a simple ring. In terms of shape, the nitrogen and methyl group create a little bulk on one side, while the ethanol stretches out, giving the molecule both flexibility and options for hydrogen bonding.
There’s a reason researchers seek out compounds like this. The nitrogen in these rings gives the whole molecule a basic character, which shapes how it reacts in the lab and in living systems. In medicinal chemistry, nitrogen rings like piperidine pop up all over the place. Adding a methyl group to the nitrogen, as seen here, sometimes tweaks how the compound interacts with enzymes or receptors because the bulk and electron distribution change.
That ethanol group isn't just a decorative flourish. It opens doors for solubility changes and lets chemists add the molecule into reactions where hydrogen bonding plays a role. Sometimes, even a simple substitution like this can mean the difference between a blockbuster drug and an inactive compound. The entire piperidine-ethanol structure allows for real versatility in organic synthesis.
Organic labs often keep compounds like 1-Methylpiperidine-2-ethanol on hand for work as intermediates. These sorts of chemicals play a role in building more complex molecules, especially ones that end up as pharmaceuticals. Drug makers, for instance, appreciate how the piperidine ring can mimic other biological shapes. The ethanol side arm offers two carbons and an alcohol group to join with other molecules or adjust solubility, fine-tuning a parent compound’s properties.
From a safety and regulatory perspective, every chemical that hits the bench carries risks. The amine group can sometimes pick up or drop off protons, changing its chemical behavior. That changes things like toxicity or environmental persistence. Industry experts know the need for tight protocols and good training to keep these reactions predictable and safe—especially since small tweaks to a chemical’s structure can shift risk levels.
Chemistry teachers and researchers push for strong foundations in chemical literacy. The story of 1-Methylpiperidine-2-ethanol reminds us how each atom and bond has a job. It speaks to the value of not just memorizing formulas, but thinking through structure, reactivity, and safety together. To truly dig into a molecule’s promise, teams need to keep up with advances in analysis and regulation, making sure skill keeps pace with innovation.
Better education, transparent research, and clear communication keep the field on steady ground. As new compounds get discovered and used in medicine or industry, the careful study of their structures—like that of 1-Methylpiperidine-2-ethanol—remains essential for healthy progress.
Some chemicals demand respect. 1-Methylpiperidine-2-Ethanol sits in that category. Its molecular structure may seem simple, but the headaches from mishandling it stretch far. My own time in a crowded university lab taught me that taking shortcuts with storage often came back to haunt us. Unlabeled bottles meant wasted reagents and sometimes half a day figuring out where a strange smell came from. When the substance carries risks of irritation, skin sensitivity, or flammability, those little details turn serious fast.
Heat speeds up chemical reactions, even those you don’t want. Leaving 1-Methylpiperidine-2-Ethanol near a sunny window or in a warm storeroom invites trouble. Its volatility means low temperatures slow vapor formation, reducing inhalation risks. A cool room—below 25°C—makes sense. Refrigerators help, but only if they’re spark-proof, since vapors from volatile substances can spark fires. In my experience, someone always tries to tuck extra bottles into a shared lunch fridge. Bad call.
Most amines react with moisture over time. Humid air can cause contamination and may even trigger side reactions, especially over weeks or months. Tight seal on the container, desiccant packs nearby, and a habit of keeping lids on, rather than letting them clatter on a shelf, go a long way. After seeing crystal growth in what should have remained a clear liquid, I learned not to skip those steps.
Some substances break down faster when exposed to light. Opaque or amber bottles best protect the contents from UV rays that sneak in through windows. In one lab I worked in, simply switching from clear glass to brown for sensitive liquids extended shelf lives by months. Investing in the right type of container pays back in reduced waste and improved safety.
Mixing storage of amines near acids or oxidizers creates a recipe for hazardous reactions. Even small accidental leaks can set off strong odors or produce gases nobody wants to breathe. I’ve seen cabinets marked for “Bases only” or “Acids only,” and the separation proved its worth time and again. Most chemical suppliers give clear guidance—following it always felt like common sense, not red tape.
Eyeballing clear liquids invites disaster, so every container should carry the chemical name, date received, expiration date, and hazard symbols. Permanent markers fade; printed labels last. Unlabeled bottles left by “just this one time” decisions have forced evacuations. It takes far less time to label upfront than to conduct a spill clean-up later.
Small leaks, even in well-sealed containers, add up. Storing volatile or pungent chemicals in cabinets with built-in ventilation can prevent workroom odors and accidental exposures. Good airflow makes a difference. After one summer spent working in a poorly ventilated storeroom, I began to appreciate companies that invest in fume hoods and specialty cabinets. The health benefits far outweigh any upfront cost.
Absorbent materials, nitrile gloves, and a written spill plan belong wherever 1-Methylpiperidine-2-Ethanol gets stored. Spills attract attention fast, and knowing where to find cleanup gear keeps the focus on the task, not on improvisation in a crisis. Lab audits don’t just check that equipment is present—they see if staff know how to use it. Practicing those steps for real-world readiness helps avoid panic if the unexpected happens.
As someone who has spent plenty of time tinkering in both home and professional labs, certain details about chemicals tend to jump out—which is especially true for 1-Methylpiperidine-2-ethanol. Labeled by its ring structure and that little ethanol extension, it appears as a colorless to slightly yellowish liquid when pure. Right off the bat, its smell carries some of that amine sharpness, not quite enough to knock you down, but you know it means business. If you’ve ever accidentally split similar piperidine compounds, you know the scent lingers for hours.
Density checks usually clock it between 0.90 and 1.00 g/cm3 at room temperature, lighter than water. Chemists who have to measure out doses know how it’s easy to draw up into a pipette, making it easier on the hands compared to thicker, oily substances. As for water solubility, the ethanol group earns its stripes: this stuff dissolves without a fight, mixing well with both alcohol and water. That means cleanup never gives much trouble, and dilutions for testing or reactions go smoothly. Boiling points usually fall in the 200–220°C range. You don’t get crazy volatility, which makes it safer during synthesis, though strong ventilation always helps when working at scale.
Mixing together a piperidine ring with a methyl at nitrogen and a two-carbon alcohol tail makes things lively. That secondary amine center and the primary alcohol set the stage for a real two-faced personality. In reactions, I’ve seen the amine act as a mild base, eager to grab up stray protons and form stable salts, especially with hydrochloric acid. So if you’re ever watching pH changes or prepping to isolate it after a reaction, expect salt formation to come into play.
The alcohol group won’t just sit on its hands either. It’ll form esters if treated with acid chlorides or anhydrides. Plenty of synthetic chemists love this dual functionality, since it lets you slot the compound into different branches of medicinal and agrochemical development. Oxidation with the right reagent can turn that alcohol into an aldehyde, giving you another building block. Its backbone stays stable under mild heat, but harsh oxidizers or acids can chew up the piperidine ring.
Seeing a compound like 1-Methylpiperidine-2-ethanol in the wild reminds me why details matter so much. Its easy handling (since it’s a liquid and dissolves without hassle) cuts down on wasted time and cleanup. That clean profile also means technicians don’t deal with sticky residues or volatile fumes that invade the workspace, which reduces exposure risk.
In pharma and laboratory circles, those two functional groups open doors. The amine and alcohol can be transformed or protected in steps, speeding up the search for new drug candidates or specialty ingredients for other industries. Researchers who focus on chemical customization find this skeleton a reliable bridge to dozens of tailored molecules, thanks to how it reacts both as an amine and an alcohol.
Every chemical has its quirks, and this one’s no exception. The sharp smell signals exposure, so using gloves and goggles feels routine. Standard fume hoods protect air quality when you’re pouring or heating the compound. Storage should be dry and away from strong oxidizing agents, since accidental mixing can’t always be fixed with a quick neutralization.
Waste handling deserves a mention. I’ve found that its solubility means you can’t pour leftovers down the drain, so special collection for organic solvents ensures safety and environmental responsibility. Regulatory guidelines keep everyone informed and reduce the chance of slip-ups, but on the ground, sharing best lab practices with newcomers makes the biggest difference.
Tweaking ventilation, meticulously labeling containers, and running spot tests before scaling a reaction all help keep mistakes from snowballing. The way forward rests on mixing common sense, respect for the compound, and a willingness to tag in technology—whether for monitoring concentration or better cleanup after the day’s work is done.
| Names | |
| Preferred IUPAC name | 2-(1-Methylpiperidin-2-yl)ethan-1-ol |
| Other names |
1-(2-Hydroxyethyl)-1-methylpiperidine 1-Methyl-2-(2-hydroxyethyl)piperidine |
| Pronunciation | /waɪˈmɛθ.əl.paɪˈpɪr.ɪˌdiːn.tuːˈɛθ.ə.nɒl/ |
| Identifiers | |
| CAS Number | 49652-55-1 |
| Beilstein Reference | 1209280 |
| ChEBI | CHEBI:189337 |
| ChEMBL | CHEMBL1805857 |
| ChemSpider | 20634907 |
| DrugBank | DB08313 |
| ECHA InfoCard | ECHA InfoCard: 100_1459 |
| Gmelin Reference | 162031 |
| KEGG | C06085 |
| MeSH | D017211 |
| PubChem CID | 12301138 |
| RTECS number | UJ5950000 |
| UNII | JU6U2XD97O |
| UN number | UN3439 |
| CompTox Dashboard (EPA) | DTXSID7076609 |
| Properties | |
| Chemical formula | C8H17NO |
| Molar mass | 129.22 g/mol |
| Appearance | Colorless to light yellow liquid |
| Odor | amine-like |
| Density | 0.916 g/mL at 25 °C (lit.) |
| Solubility in water | Slightly soluble |
| log P | 0.2 |
| Vapor pressure | 0.26 mmHg (25°C) |
| Acidity (pKa) | pKa = 10.1 |
| Basicity (pKb) | 5.78 |
| Magnetic susceptibility (χ) | -63.93×10^-6 cm³/mol |
| Refractive index (nD) | 1.439 |
| Viscosity | 0.852 cP (20°C) |
| Dipole moment | 2.33 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 369.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -219.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4627.8 kJ/mol |
| Pharmacology | |
| ATC code | N07XX11 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation, may cause respiratory irritation |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H226, H302, H314 |
| Precautionary statements | P261, P280, P301+P312, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 77 °C |
| Lethal dose or concentration | LD₅₀ (oral, rat): 870 mg/kg |
| LD50 (median dose) | LD50 (median dose): 670 mg/kg (rat, oral) |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 1-Methylpiperidine-2-Ethanol is not specifically established by OSHA. |
| REL (Recommended) | 10 mg/m3 |
| IDLH (Immediate danger) | NIOSH has not established an IDLH for 1-Methylpiperidine-2-Ethanol. |
| Related compounds | |
| Related compounds |
1-Methylpiperidine 2-Ethylpiperidine Piperidine N-Methylpiperidine 2-Piperidinemethanol |