Chemists often stumble upon molecules with unexpected staying power. 1-Methylpiperidin-4-ol has a backstory dating to the middle decades of the twentieth century, back when researchers grew curious about morpholine and piperidine analogues. In those years, new piperidine derivatives meant potential for synthesizing medicines that could treat pain, act as central nervous system stimulants, or serve as critical building blocks for agricultural chemistry. The unique simplicity and reactivity of the piperidine ring, blended with the subtle nudge from a methyl group and a hydroxyl at specific positions, made 1-Methylpiperidin-4-ol a molecule for experimentation in therapeutic research, especially for exploring new local anesthetics, antispasmodics, and pharmaceutical intermediates.
At first glance, 1-Methylpiperidin-4-ol shows itself as a clear to pale yellow liquid or sometimes as a solid, depending on temperature and purity. With a formula of C6H13NO and a molecular weight of 115.18 g/mol, it offers chemists a scaffold that adapts to innovation. Its presence in labs goes beyond just being a chemical shelf name. Scientists rely on its versatility for modifying larger molecules, improving solubility, or tweaking biological activity. Whether as a precursor for drugs or as an intermediate for agrochemical development, its practical uses grew steadily throughout the late twentieth century and now span across fields.
This compound blends water solubility with a mild, amine-like odor that gives away its family history. The melting point hovers around 26-28°C, making it sensitive to changes in lab temperature, which sometimes causes it to fluidize unexpectedly. The boiling point rarely exceeds 200°C under atmospheric pressure, and vapor pressure demands adequate ventilation during workups. The structure features a six-membered, nitrogen-containing ring, carrying a methyl group at the 1-position and a hydroxy group at the 4-position, creating distinct polarity shifts that affect its reactivity and handling. The hydroxyl group lends itself to hydrogen bonding, pulling the molecule into solubility assays and reaction screens for potential pharmaceutical derivatives.
Technical specifications for 1-Methylpiperidin-4-ol depend on the intended scale and end use. Pharmaceutical grades require purity levels south of 99%, with careful monitoring for residual solvents and heavy metals, while industrial lots may accept a narrower threshold for trace impurities, focusing mainly on water content and amine integrity. Labeling standards demand clarity on hazard identification, featuring the GHS pictogram for skin and eye irritancy, and instructions on protective gear. Packaging typically involves amber glass for laboratory use, given the light sensitivity, and HDPE containers for larger quantities, always sealed to prevent atmospheric moisture entry and limit volatilization. Standard storage advice rests on cool, well-ventilated shelves, away from oxidizers.
Many synthetic schemes pivot on reductive amination or selective alkylation pathways. Cyclization of appropriate diamine precursors, followed by N-alkylation with methylating agents, yields the core structure. One classic approach harnesses 4-piperidone, which, after methylation and reduction, delivers 1-Methylpiperidin-4-ol with decent yield. The process can call for catalytic hydrogenation—either over palladium-on-carbon or Raney nickel—as well as control of heat and moisture. Tackling stereochemistry also factors in, since uncontrolled conditions might skew the production of side-ring products or result in over-alkylation. Synthetic efficiency keeps improving, especially with new transition-metal catalysts and green chemistry initiatives reducing reliance on hazardous solvents or excess reagents.
The molecule’s nitrogen enables alkylation, acylation, and quaternization reactions, expanding the range of possible derivatives for drug design or material science. The secondary alcohol opens doors for esterification, oxidation, or substitution. Lab practitioners often transform the alcohol into an ester or a carbamate to study pharmacokinetic properties, or oxidize it for new ring analogues. Its presence in multicomponent reactions adds value, as the scaffold tolerates modifications without ring cleavage, facilitating scaffold hopping in medicinal chemistry. Presence of both nucleophilic and electrophilic sites renders it a convenient “handle” for developing higher-functionality libraries or screening new reaction mechanisms that feed into commercial applications.
Catalogs list varied synonyms, including N-Methyl-4-hydroxypiperidine, 1-Methyl-4-piperidinol, and sometimes simply 4-Hydroxy-1-methylpiperidine. Regulatory documents will mention CAS numbers—6687-19-6—and batch-specific identifiers depending on manufacturer. Larger chemical suppliers log these names under “piperidine derivatives” for custom synthesis, while pharmaceutical companies tuck the compound under internal codes that often change as research programs progress, complicating cross-referencing for new researchers.
Handling this compound brings moderate risk; like many piperidines, it may cause irritation on contact with skin or eyes and can be harmful if swallowed or inhaled in vaporized form. Laboratory teams rightly emphasize gloves, goggles, and lab coats. Fume hoods become essential during larger-scale operations. Material Safety Data Sheets recommend storing away from incompatible chemicals, like strong acids, oxidants, and nitrosating agents. First aid involves rinsing with water in case of contact, and medical attention for persistent symptoms. Chronic exposure studies remain sparse, so ongoing vigilance matters. Regulatory agencies don’t list it as especially hazardous, but regular audits and up-to-date training ensure safe practices.
1-Methylpiperidin-4-ol doesn't stay stuck in a single role. In pharma, it's a backbone for antihistamines, analgesics, or even certain psychotropic agents. Agrochemical formulators leverage its reactivity to craft pest control molecules with bioselectivity. Research chemists in academia use it for library synthesis—spawning novel compounds by tweaking the base scaffold. Its structural motif makes it a popular intermediate in the search for more potent, safer, or more selective molecular targets. Beyond the chemical and pharmaceutical labs, specialists in material sciences experiment with its functional groups to develop new polymers or surfactants, seeking improved resistance or tailored functionalization.
Current R&D teams explore greener production methods—shifting toward enzymatic synthesis or flow chemistry to cut down on waste and boost yield. Interest remains high in finding piperidine derivatives that cross the blood-brain barrier more effectively for neurological drug development. Recent efforts try to optimize modifications at the ring’s hydroxyl position, influencing not just potency but metabolic stability, aiming for safer and longer-acting formulations. Open-access platforms track new patents on 1-Methylpiperidin-4-ol derivatives, showing a rising trend in combinatorial chemistry uses. Universities and private labs collaborate with manufacturers to scale up synthesis, cut prices, and ensure supply chain resilience, which became crucial after disruptions in global logistics.
Available toxicology profiles say acute exposure's main risks involve mucosal irritation and mild central nervous system depression, similar to other low-molecular-weight amines. Rodent studies haven’t found carcinogenicity at experimental doses, but chronic toxicity data still needs expanding. The metabolite profile suggests most is excreted unmetabolized or after conjugation with glucuronic acid. This elimination route keeps bioaccumulation concerns low, but researchers advocate for monitoring as usage grows in new industries. Environmental studies test for breakdown in soil and water; early results show moderate persistence, raising concerns in case of industrial spills. Waste treatment protocols use oxidation and adsorption to remediate risks.
Years in laboratories haven’t dulled its potential. The push for personalized medicine increases demand for easily modifiable ring systems like this one. As machine learning tools speed up screening of new analogues, 1-Methylpiperidin-4-ol stands out for its simplicity and flexibility. More sustainable production processes aim to shrink carbon footprints, with researchers trialing biocatalysts and solvent-free procedures. There’s growing hope for environmental monitoring methods that rely on derivatives of this molecule, especially in sensors or test kits. Regulatory harmonization across global suppliers could streamline approval for new therapeutics. Future work needs to clarify chronic exposure impacts, unlock higher-value reactions, and develop safer, scalable syntheses that don’t sacrifice purity or performance.
Lab benches always seem filled with odd-sounding names—1-Methylpiperidin-4-Ol pops up now and then. Not a household substance, but ask folks in pharmaceuticals, and they’ll tell you it’s got clout. The world often focuses on the miracle pills and therapies instead of what goes on before a finished tablet hits a pharmacy shelf. That’s where building block chemicals take center stage, and 1-Methylpiperidin-4-Ol falls firmly into that category. People in chemical synthesis value it for being a handy starting piece that links into bigger, more complex molecules.
I’ve worked behind the scenes in labs, watching researchers chase after new treatments for old problems. The hunt for better drugs involves a ton of trial, error, and clever chemistry. 1-Methylpiperidin-4-Ol plays a role here as a basic structure for active pharmaceutical ingredients (APIs). Think of it as a Lego block with the right shape, ready for a scientist to click onto something else. It pops up in pathways that lead to various medications, including some antihistamines and painkillers. Piperidine rings—like the one inside this molecule—turn up in therapies for everything from depression to hypertension.
The pharmaceutical industry tasks itself with chasing safety and performance. Repeated testing, endless paperwork, and never-ending tweaks to molecular design require a library of building blocks. 1-Methylpiperidin-4-Ol helps fill out that library, giving chemists a reliable scaffold to modify and improve molecules for human use. Companies pouring investments into drug discovery look for stable, well-understood chemicals, and this one checks that box. Its history in drug synthesis textbooks earns it a space on most chemical suppliers’ shelves for a reason.
Pharma isn’t the only field leaning on this substance. The fragrance industry grabs onto piperidine derivatives to stitch together new scents—sometimes, a molecule like this adds complexity or longevity to a product. I’ve written copy for a specialty chemical supplier and noticed the way cosmetic chemists seek out these odd compounds to sculpt everything from lotions to hairstyling products. The ability to tweak molecular structure gives manufacturers broader creative choices and can help beat competitors with products that work just a little better or last a bit longer.
A less talked about use comes from the world of intermediates for fine chemicals. Academic research might look ordinary from the outside, but inside, teams are racing to invent dyes, polymers, or even quantum computing materials. 1-Methylpiperidin-4-Ol serves as a stop along the synthetic pathway—add a group here, snip something off there, bend it in just the right way, and the new molecule takes shape. Eventually, someone’s innovation improves lives, even if the starting ingredient never gets its time in the spotlight.
Handling lab chemicals has always carried risks. People working with 1-Methylpiperidin-4-Ol watch out for possible toxicity, as some piperidine derivatives can irritate skin and eyes or lead to other hazards. Factories investing in safety improvements don’t just tick boxes for compliance—they protect workers and avoid incidents. Organizations like OSHA and REACH push for careful handling, clear documentation, and improved training so these useful chemicals don’t end up causing harm.
Innovation keeps marching, fueled by humble intermediates like 1-Methylpiperidin-4-Ol. Whether it winds up in the next painkiller or adds backbone to a new scent, the real value shows up in the problem-solving mindset of chemists and engineers. Stepping into the future, these basic compounds give researchers options—and with the right safeguards, they drive the discoveries that matter most to our health and daily life.
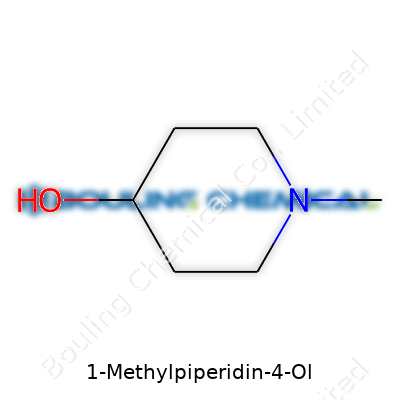
On paper, 1-Methylpiperidin-4-ol catches some folks off guard. The name sounds straight out of a medicinal chemistry class, yet its structure tells a basic story. The backbone comes from piperidine, a six-membered ring with one nitrogen and five carbons. These kinds of rings show up all over the place in both pharmaceuticals and organic synthesis. The “1-Methyl” part means a single methyl group attaches right at the nitrogen. Over at carbon number four, an -OH group sits prominently—that's where "4-ol" kicks in, showing the presence of the alcohol function.
I’ve worked in labs where piperidine rings get thrown around all the time, especially for intermediate steps in drug development. With 1-Methylpiperidin-4-ol, adding that methyl to the nitrogen changes a lot about how it acts compared to the parent molecule. Even tiny switches like this change both its reactivity and how it might fit into bigger molecular scaffolds.
Let’s walk through it: Start with a hexagonal ring—five carbon atoms and one nitrogen. Picture carbon 4 carrying a hydroxyl group. The nitrogen at position 1, instead of keeping its lone hydrogen, now takes a methyl group (CH3). Chemists usually draw this so the methyl and hydroxyl sit far from each other, keeping the molecule’s 3D shape clear. Structurally, it looks like this:
This structure isn't just for show. Piperidine derivatives form core elements in a lot of medicines, helping them interact well with the human body because the ring itself mimics certain neurotransmitters. 1-Methylpiperidin-4-ol stands out for its own uses, whether as a synthetic intermediate or building block for more complex compounds.
Getting the structure right has ripple effects beyond textbooks. I’ve seen instances where subbing a methyl group makes the difference between a compound that dissolves and one that stays chalky. With 1-Methylpiperidin-4-ol, the combination of the alcohol and the methyl-nitrogen softens its basicity, improves water solubility, and regulates how it fits into receptor sites. It serves as a handy stepping stone for synthesizing drug candidates and research chemicals. Just in the past few years, modifications on this backbone have appeared in multiple patents for new pharmaceuticals.
From a safety standpoint, knowing exactly where the methyl and hydroxyl land helps avoid surprises during experiments. Chemical reactions sometimes take odd turns if a functional group is out of place. By clearly mapping out the structure of 1-Methylpiperidin-4-ol, mistakes drop, and yields climb higher. This sort of precision matters to anyone scaling up production from grams to kilograms.
Every time a group in the lab tries a new synthesis with molecules like this, they look for improved yields, fewer side products, and easier purifications. Advances in spectral analysis tools, such as NMR and mass spec, give clear confirmation of the methyl and hydroxyl placement for 1-Methylpiperidin-4-ol. Better access to verified reference materials helps chemists confirm their structures more easily and prevents wasted time rerunning tests.
Along with technology, improved training for lab workers also pays off. Making sure everyone, from interns to senior staff, recognizes how a methyl group on nitrogen or an alcohol on carbon four changes properties means greater consistency. Reliable structure means safer, more predictable chemistry. That step—from knowing the skeleton of the molecule to understanding its real-world usefulness—is what lifts the field ahead.
Chemicals like 1-Methylpiperidin-4-ol don’t usually make the evening news, but they quietly shape how things run in production labs around the world. Anyone who’s poured from a bottle with a tongue-twisting name like this knows: it’s not just the hazard labels that matter. Real safety depends on a mix of preparation, equipment, and street smarts learned through experience.
Working with 1-Methylpiperidin-4-ol means paying attention. Its structure lands it in a class of organic substances that tend to raise red flags for their toxicity. Scientists classify it as hazardous because it irritates the skin and eyes. One drop, even in a well-ventilated workspace, causes burning sensations. Accidental contact with eyes triggers pain fast; washing out right away becomes a race against time.
If you ever took a deep breath near a volatile amine and felt your eyes water or your throat itch, it’s not a stretch to see this chemical joining that club. Small studies point to harmful effects in rodents after exposure, but humans haven’t been studied in the same way. Factories storing or processing this compound stick with safety measures learned from dealing with similar substances.
Every bottle comes with information sheets warning about gloves, goggles, fume hoods, and emergency rinses. These details save people from miserable days in the health office. Gloves like nitrile stand between your skin and dangerous absorption. Lab coats keep splash zones off regular clothes; goggles defend the eyes, your most sensitive target for these splashes.
Spills happen, especially when rush jobs or late nights eat away at focus. Teams with a culture of double-checking labels and spill kits in reach cut down on accidents. Sharing stories about close calls does more for safety than walls of regulatory posters.
Researchers don’t work in the dark. Sources like the National Institute for Occupational Safety and Health (NIOSH) and the European Chemicals Agency provide hazard summaries and storage instructions. Public records show a lack of long-term data for 1-Methylpiperidin-4-ol, so workers default to treating it like stronger amines—never trusting appearance to guess at safety.
There’s no substitute for on-the-ground experience. More seasoned lab mates pass down shortcuts, like storing it away from open flames and always labeling beakers, even in short-term projects. Clean air remains essential. Fume hoods and working in well-ventilated areas stop vapors from making problems bigger than a minor skin rash.
Companies that get ahead focus on creating an environment where it’s easy for workers to raise concerns. It pays to keep safety equipment in top shape and run drills for chemical spills. It’s tempting to rush, but taking the extra minute to clean up, label, and check your protective gear keeps people in the lab and out of the hospital.
For anyone handling chemicals, including 1-Methylpiperidin-4-ol, safety works best as a habit, not a box to check. The goal: everyone goes home at the end of the day, no matter how routine the job seems.
1-Methylpiperidin-4-ol comes up more in pharmaceutical and chemical research than most realize. The compound grabs attention because its structure, a piperidine ring with a methyl and a hydroxy group, makes it popular for building complex molecules. Yet, something as simple as the molecular weight—one of the first facts every chemist checks—often gets brushed aside after the first calculation. The actual value shows up again and again, driving formula weights, stoichiometry, and operating as a bridge between tiny lab scales and full industrial batches.
The molecular weight for 1-Methylpiperidin-4-ol clocks in at 115.18 grams per mole. This value flows from the molecular formula: C6H13NO. To break it down, that means six carbon atoms, thirteen hydrogens, one nitrogen, and one oxygen. Calculators and chemical software spit out this number in seconds, but before the digital age, working it out by hand required memorizing atomic weights. It matters more than just on paper; the number decides how much reagent to weigh, how much solvent to use, and how much product to expect from a synthesis.
Minor slip-ups with molecular weight send ripples through experiments. I’ve seen labs lose days because of a slip on the calculator or a hasty copy-paste from a supplier’s outdated datasheet. Production planners, weighing grams or kilograms, need a number rooted in proper calculations or validation, not rounded-off estimates. Even a decimal-point drift in the molecular weight can leave a chemist wondering where yields disappeared or why purity drops off.
Imagine prepping a 0.1 mol solution of 1-Methylpiperidin-4-ol. With the right figure, weighing out 11.52 grams lands right on target for 100 milliliters. Slip in a wrong value—maybe from an isomer or an impurity—and the solution goes off. Over months of repeated measurements, all of these slips add up. This doesn’t just change reaction rates or yields. In tight regulatory regimes, like pharmaceutical manufacturing, inconsistent batches can lead to failed quality-control checks. I once watched a regulatory audit pick up on a 1% variance large enough to stop an entire production run cold.
Lots of chemists look for confirmation from open chemistry databases or trusted supplier catalogs before landing on any formula. I rely on sources like the NIST Chemistry WebBook or PubChem for confirmation. One bad source could skew a project’s raw cost estimate or dosing limits. In classrooms, instructors bake these checks into every assignment. In professional labs, cross-referencing data with certified software or repeating the lookup by hand insulates a team from hasty errors.
It takes more than published numbers to guarantee consistency. Advocating double-verification of every reagent’s molecular weight seems basic, but it saves time and money in the long run. Some labs standardize a set procedure for every new batch: compare supplier specs, then recheck them in open databases and match them against in-house reference materials. Digital lab notebooks help catch changes when a supplier updates entries or a new isomer gets discovered, putting every calculation in one record for the entire team to track.
Many labs and chemical manufacturers depend on 1-Methylpiperidin-4-ol. This compound sits on the shelves of research facilities where the stakes run high for safety. Its chemical profile shows a slightly greasy texture, a faint odor, and good solubility in water. These features already hint at some practical steps for storage and handling. Experience in the lab has taught me never to judge a bottle just by what's on the label—subtle hazards hide in plain sight.
Direct light and open air aren't friends of this compound. Exposure can trigger degradation or introduce impurities. I've worked in spaces where light-sensitive bottles turn strange colors after a few careless days on the benchtop. Amber glass containers cut down the risk, blocking light that can speed up chemical changes. Tightly sealing the lid on the container keeps oxygen and moisture out, protecting the contents and, more importantly, everyone working in that area.
Room temperature in most labs drifts from cool to a touch warmer, but it's worth setting a standard. 1-Methylpiperidin-4-ol keeps its integrity best at a stable, cool temperature—around 2–8°C, like a dedicated chemical refrigerator. Store it somewhere free from temperature spikes. Sudden warming or cooling stresses the bottle and its contents, and you risk condensation or crystal formation on the inside walls. Even minor container sweating can mean moisture's crept in, which might throw off both purity and performance. That's something anyone running syntheses or preparing reagents doesn't want.
I've learned not to leave bottles near the edge of a countertop or anywhere that gets crowded. Even cautious scientists bump their elbows now and then. The risk of spilling 1-Methylpiperidin-4-ol carries consequences—skin and eye irritation tops the list. Goggles and gloves make sense both in theory and practice. Labeling grows more important the older and fuller the stockroom gets. Use clear, chemical-resistant labels that show the date received, opened, and who handled it last. These details make a difference during audits or inspections and speed up disaster response if somebody drops the bottle.
Chemicals with a noticeable odor or potential vapor hazards belong in a ventilated, designated storage cabinet. Some colleagues treat this as overkill, but a single whiff of a noxious vapor in a cramped storeroom convinced me otherwise. Segregate 1-Methylpiperidin-4-ol from oxidizers and strong acids to dodge dangerous reactions. Even the most careful person can slip up, so physical separation adds a true layer of precaution.
Good storage habits grow from routine training and clear protocols. Everyone, from new recruits to seasoned techs, benefits from refresher courses on chemical safety. Invest in readable, up-to-date safety data sheets and post them close to storage spots. Keep eye wash stations and spill kits within arm’s reach. Setting up checklists for weekly inspections catches expired containers and keeps storage conditions optimal. I've found that sharing eye-opening stories about near misses motivates teams much more than safety posters alone.
Every bottle of 1-Methylpiperidin-4-ol brings both opportunity and risk. Consistent, mindful storage reflects a shared responsibility. Take care of the details—proper containers, labels, climate, and training—because these steps keep people safe, protect research, and show respect for everyone who works with or near these chemicals. Storing this compound right isn’t just about compliance; it builds real trust between team members and sets a solid example for how science should be done.
| Names | |
| Preferred IUPAC name | 4-Methylpiperidin-1-ol |
| Other names |
4-Hydroxy-1-methylpiperidine 1-Methyl-4-piperidinol 1-Methyl-4-hydroxypiperidine NSC 37754 |
| Pronunciation | /ˈwʌn ˈmɛθɪl paɪˈpɛrɪdɪn fɔːl/ |
| Identifiers | |
| CAS Number | 625-76-5 |
| 3D model (JSmol) | `3Dmol 'C1CCN(CC1)CO'` |
| Beilstein Reference | 1333351 |
| ChEBI | CHEBI:89350 |
| ChEMBL | CHEMBL402060 |
| ChemSpider | 160082 |
| DrugBank | DB08365 |
| ECHA InfoCard | ECHA InfoCard: 100_009_800 |
| EC Number | 223-002-7 |
| Gmelin Reference | 82144 |
| KEGG | C18935 |
| MeSH | D018233 |
| PubChem CID | 125973 |
| RTECS number | UY4375000 |
| UNII | 027T2FLQ21 |
| UN number | UN3439 |
| Properties | |
| Chemical formula | C6H13NO |
| Molar mass | 115.19 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | amine-like |
| Density | 0.96 g/cm3 |
| Solubility in water | soluble |
| log P | 0.13 |
| Vapor pressure | 0.185 mmHg (25°C) |
| Acidity (pKa) | 10.26 |
| Basicity (pKb) | pKb = 3.38 |
| Magnetic susceptibility (χ) | -61.26·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.450 |
| Viscosity | 1.1 mPa·s (20 °C) |
| Dipole moment | 2.33 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 318.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -180.3 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4334.3 kJ/mol |
| Pharmacology | |
| ATC code | N04BX11 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P280, P302+P352, P305+P351+P338, P312, P337+P313, P362+P364 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 84°C |
| Autoignition temperature | 280 °C |
| Lethal dose or concentration | LD50 (rat, oral): 670 mg/kg |
| LD50 (median dose) | LD50 (median dose) of 1-Methylpiperidin-4-Ol is "325 mg/kg (rat, oral) |
| NIOSH | JN8225000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 1-Methylpiperidin-4-Ol is not specifically established by OSHA. |
| REL (Recommended) | 0.25 ppm (as string) |
| Related compounds | |
| Related compounds |
4-Piperidone Piperidine 1-Methylpiperidine 4-Hydroxypiperidine |