Big strides in organic chemistry often come from a deeper look at simple rings, and that rings true for 1-Methylpiperazine. Its story traces back to the rise of heterocyclic chemistry in the early twentieth century. Researchers chasing after effective drug scaffolds and industrial solvents started paying closer attention to compounds like piperazine. The methylated derivative, 1-Methylpiperazine, emerged from sustained tinkering in academic and pharmaceutical labs. Decades ago, chemists needed cleaner, more stable cyclic amines. Compared to other candidates, the introduction of a methyl group improved both stability and versatility, opening new avenues in synthesis and formulation. Companies started ramping up production when demands in the pharmaceutical and agrochemical industries peaked in the late 1960s. Patent filings began to snowball, and more nuanced preparation methods appeared by the 1980s as both academia and industry looked for ways to improve reaction specificity and yield.
1-Methylpiperazine stands out as a colorless liquid or crystalline solid with a mild, amine-like odor. Its use covers all corners of chemical manufacturing, R&D, and pharmaceutical development. As my lab experience shows, this molecule strikes a balance between reactivity and stability that most synthetic chemists appreciate. It's common to spot 1-Methylpiperazine on the ingredient lists of drug intermediates, corrosion inhibitors, and even specialty coatings. Drug developers tap into its ring for making antipsychotics, antidepressants, and other nitrogen-containing scaffolds that reach clinical trials. Its solvent properties also benefit reactions that need water-miscible amines.
Pouring out of a reagent bottle, 1-Methylpiperazine reveals a boiling point close to 138-140°C and a melting point near -1°C. Its density hovers around 0.92 g/cm³. The compound dissolves well in water, ethanol, and most polar organic solvents; that makes it handy for processes demanding liquid-liquid extractions. Chemically, 1-Methylpiperazine comes with a secondary amine and a methyl group, giving it a basicity on par with similar substituted piperazines. The N-methyl group adds a bit of lipophilicity, which tweaks its partition behavior and reactivity with both electrophiles and nucleophiles. Its vapor pressure remains moderate, and left open, it can absorb moisture and CO₂ from air, leading to potential amine carbonate formation. Stability under ambient conditions works to the chemist’s favor, since storage risks remain low with proper sealing.
Commercial suppliers offer 1-Methylpiperazine at purities that often exceed 98%. Labels on each container lay out hazard codes with familiar pictograms for flammability and health, accompanied by batch numbers and shelf life estimates. Documented MSDS sheets specify routes of exposure and emergency guidance. Storage instructions highlight the need for tight closures, cool settings, and good ventilation. Some batches get certified for trace metal content or pharmaceutical-use restrictions, especially where end-users need consistency for sensitive syntheses. Each lot gets tracked for color, water content by Karl Fischer titration, and a broad scan using NMR and GC-MS for impurities. Labs and factories keep a close eye on these details, because minor contaminants can influence yield or unwanted side-reaction profiles.
Making 1-Methylpiperazine calls for a shaded corner of alkylation chemistry. Labs lean most often on the direct methylation route, starting with piperazine and methylating agents like methyl iodide or dimethyl sulfate. Strong bases, usually K₂CO₃ or NaOH, trigger the reaction. The choice falls between one-pot synthesis and stepwise routes, but one-pot methods save cleanup and cut costs. Industrial plants prefer batch or continuous processes, using reaction vessels designed to handle volatile methylating agents and high temperatures. Operators carefully monitor temperatures to dodge side reactions and limit poly-alkylation that would degrade purity. Purification includes solvent extraction and vacuum distillation, followed by drying with molecular sieves to satisfy water content specs.
1-Methylpiperazine’s chemistry goes beyond simple methylation. Its nitrogen atoms serve as nucleophilic centers, attacking alkyl halides, acylating agents, and sulfone donors. Electrophilic aromatic substitutions and ring-opening add layers of complexity. Cross-coupling reactions—think Suzuki or Buchwald-Hartwig—find good partners in this molecule, especially when researchers need to attach aromatic scaffolds. More specialized groups leverage it as a ligand in metal-catalyzed transformations. Because of its cyclic structure, modifications on the N4 or C3 site can turn up new chemical and biological profiles. Reductive amination and N-oxidation further diversify downstream possibilities. Synthetically, this molecule is a toolbox staple when building advanced heterocyclic frameworks.
In catalogs, 1-Methylpiperazine appears under several aliases, reflecting its wide adoption. You’ll sometimes find it listed as N-Methylpiperazine, methylpiperazine, or 1-Methyl-hexahydropyrazine. Internationally, the same product might show up on shipping manifests as NMPZ, or follow IUPAC conventions with 1-methylpiperazine. Distributors package it under proprietary or branded labels, and each end-use sector—be it pharma, agrochemical, or chemical—sticks to its own common naming traditions.
Handling 1-Methylpiperazine raises pretty standard safety flags seen with secondary amines. Prolonged skin or eye contact triggers irritation, sometimes severe, and inhalation of vapors can cause dizziness or respiratory discomfort. Labs and production lines install hoods, provide nitrile gloves, and fix eyewash stations close by. In the event of spills, teams rely on absorbent pads and neutralizers before sending waste for certified disposal. Worker training covers the usual: keeping storage containers sealed, labeling secondary bottles to avoid mishaps, and documenting incidents for compliance audits. Regulatory authorities in North America, Europe, and Asia each put their own spin on handling, but a throughline exists—clear labeling, accessible MSDSs, and periodic safety drills. Fire risks do exist, given its moderately low flash point, and facility operators stress the presence of class B fire extinguishers in storage zones.
1-Methylpiperazine supports a vast stretch of industries. The pharmaceutical field leans on it when assembling antipsychotic and antidepressant drugs, many of which depend on substituted piperazines for receptor targeting. Water treatment companies value its strength as a corrosion inhibitor: a dose in a cooling tower or pipeline can keep rust and scale at bay. More recently, research labs started employing it in organic electronics, where electronic bandgap tuning is crucial. Its behavior as a low-toxicity, polar aprotic solvent meshes well with adhesive manufacturing, resins, and coatings that resist harsh environmental factors. In agricultural chemistry, the compound helps create fungicides and plant growth promoters. Students working through their first multistep syntheses quickly learn 1-Methylpiperazine eases purification steps and leaves behind fewer by-products compared to less-selective amines.
Most recent studies zero in on novel drug candidates that start with piperazine derivatives. Scientists scan the structure-activity relationships that 1-Methylpiperazine can open for new treatments. Researchers at large pharma outfits and academic labs churn out new analogs that might treat neurological disorders, and computational chemists run virtual screening for high-affinity protein binding. Industrial R&D teams dive into formulations where this compound boosts yield or product shelf life. Across sectors, there’s movement toward sustainable synthesis, optimizing green methylation routes or direct catalytic methods to minimize waste. Conferences and patent filings spotlight not only the molecule’s role in traditional applications, but also emerging uses in control-release fertilizers, advanced polymers, and energy storage devices. For every new field, teams work to tweak the building block for exacting performance.
Investigating the toxic profile of 1-Methylpiperazine gives way to detailed assays on cell lines, animal models, and environmental simulations. Acute exposure studies show low to moderate toxicity, with the threshold for irritation sitting higher than many comparable amines. Long-term and high-dose tests, though, do reveal signs of nephrotoxicity and mild neurotoxic effects, warranting careful dosage control. Regulatory submissions include teratogenicity and mutagenicity panels; so far, negative or near-trace effects help prop up its reputation as a relatively safe scaffold. Environmental scientists scrutinize its breakdown products—under light or oxidizing conditions, these derivatives degrade steadily, but mismanaged disposal can taint groundwater and aquatic life. That's why disposal needs strict monitoring. Ongoing clinical reviews and international safety boards keep a close watch for adverse event trends, channeling data back into workplace limits and public health directives.
Opportunities for 1-Methylpiperazine keep cropping up as chemists and engineers invent new applications. Green chemistry initiatives inspire a fresh look at sustainable production, where companies invest in waste-minimizing methylation and recycling spent solvents for closed-loop systems. Drug developers remain hungry for versatile scaffolds, and 1-Methylpiperazine still pulls weight in preclinical libraries. Materials science counts on its adaptable reactivity for new polymers and functional coatings. As technology branches into smart materials, the odds rise that N-substituted piperazines will anchor new sensor arrays or biomedical devices. With increasing data on safe handling and environmental stewardship, future standards can balance industrial productivity with occupational and ecological care. Markets like Asia Pacific, previously trailing in scale, are gearing up as capacity and R&D investment rise. The story of 1-Methylpiperazine isn’t finished—each advance in synthesis, application, and toxicology writes another chapter.
Pharmaceutical work draws on a series of small building blocks that few people ever see outside a lab; 1-methylpiperazine counts as one of these behind-the-scenes players. Chemists often reach for it to help shape molecules into new drugs. It contains a simple structure—a six-member ring with one nitrogen broken away and replaced by a methyl group. This tweak helps researchers design compounds that stick around longer in the body or fit especially well into particular receptors. I’ve worked with teams where a touch of 1-methylpiperazine altered the way a drug candidate handled water, changing how effective a pill could be. Think anti-cancer therapies or certain psychiatric medications—the foundation can start here.
Some chemical components remain hidden inside products that rarely cross our minds. 1-Methylpiperazine helps companies create materials for everyday use. For example, it acts as a curing agent in polyurethane coatings and adhesives. Car parts, flooring, or even the paint on a bike could rely on this chemistry. Without it, the strength and durability of countless items would drop. In industrial settings, teams use it to tweak the flexibility and resistance of finished materials. I’ve seen how this single choice between curing agents changes performance in everything from foam insulation panels to water-resistant sealants.
Research labs often chase the next leap in technology. 1-Methylpiperazine forms part of that chase—rarely in the headlines, but always in the background, making complicated molecules and processes possible. Scientists use it to build advanced corrosion inhibitors, making engineering more reliable and safe. Without corrosion control, factories and vehicles face costly breakdowns far sooner. I’ve heard plant managers complain about the cost of shutdowns that could be prevented by just such chemical investments.
A chemical like 1-methylpiperazine brings benefits, but it also demands respect and caution. Those who work around it, whether in pharmaceutical lines or industrial plants, need proper training and protective gear. Its vapors irritate eyes and skin, and spills prompt immediate cleanup. I remember strict safety rules in place, meant to keep workers from harm, and the layers of oversight needed for anything that enters medicine or daily products. Every chemical has risks, but proper procedures and responsibility limit accidents.
Wider concerns reach beyond worker safety. Responsible chemical management means tracking how these substances leave facilities and ensuring waste doesn’t enter the wider environment. Some countries now tighten rules on solvents and chemical precursors, looking at toxicity and persistence. In labs I’ve worked with, routine checks and clear labeling remain non-negotiable, as public health and environmental safety depend on vigilance.
Demand for tailored pharmaceuticals and more durable materials continues to grow. 1-Methylpiperazine stands as a small but vital part of the machinery that supports innovation. While its use helps create life-improving drugs and tougher everyday products, companies and scientists need to stay accountable, balancing progress with care for people and the environment. For anyone involved with chemicals, learning the ins and outs of substances like this remains essential—not just for better results, but for staying safe and responsible in a rapidly changing world.
Everyday work with chemicals often leads to less public conversation about what these compounds do to people over years of handling. 1-Methylpiperazine falls into this category, popping up in labs, pharmaceutical work, and even some cleaning agents. Its amine smell alone tips off the senses that this is not a substance anyone would want on their hands or close to their face. So the question stands out: Does 1-Methylpiperazine pose any special hazard or hidden risk?
Contact with 1-Methylpiperazine usually doesn’t happen on streets or in pantries. People working in synthesis labs or manufacturing see this compound in its pure state, and that’s where trouble shows up. Try skin contact, and real irritation starts quick—redness, itching, maybe even chemical burns in a bad case. Inhaling vapors is no better, as the nose, throat, and lungs dislike its pungent fumes. Many people talk about headaches or coughing spells in an area with poor ventilation. Companies handling this substance regularly report the need for rubber gloves, splash goggles, and tight fume hoods just to stay ahead of the risk. The facts tell a story: this is no mild-mannered chemical.
Take a look at safety datasheets and the answers follow. Studies on lab animals draw out the risk of acute toxicity from swallowing or heavy inhalation. Mice and rats exposed to concentrated forms suffer from respiratory tract damage, central nervous system effects, and sometimes death at doses not too far off from what a spill might deliver. The numbers speak for themselves—ingestion of just a few milliliters in an animal model produces serious distress. Regulatory guides label the chemical as harmful if swallowed, toxic if inhaled in strong enough doses, and corrosive to eyes and skin. Most chemical suppliers flag it as a compound worth careful respect.
Personal experience sharpened my sense of risk. I once spent an afternoon in a research lab working with piperazine derivatives. A coworker nicked his glove, then absentmindedly touched his cheek. It didn’t take long—a stinging patch of skin, some swelling, and a trip to the infirmary. Even with solvent-resistant gloves, splashing or small mistakes brought trouble. Even short stints around an open container reminded me why those strong fumes demand caution. No one needed a reminder after that about keeping protective gear in top shape.
Where 1-Methylpiperazine turns up in manufacturing or research, training stands out as the best defense. Teams who know the symptoms of overexposure act faster to rinse, ventilate, and report spills. Regular checks on ventilation systems and emergency eyewash stations save time when an accident hits. Most accidents I've seen came less from the chemical itself, and more from small slip-ups, skipped measures, or old gloves. Supervisors who push for routine walk-throughs pick up problems early, before anyone gets hurt.
Even for chemists with decades under their belts, these reminders carry weight. No shortcut or “just this once” approach belongs in labs handling substances as active and stubborn as 1-Methylpiperazine. Fresh gloves, working hoods, and honest talk about near-misses all help keep teams working safely with a chemical that brings both promise and risk to the workbench.
Ask any chemist about 1-Methylpiperazine and they’ll tell you its chemical formula is C5H12N2. This isn’t just academic trivia. That simple string of letters and numbers tells a much bigger story, one that ties together theory, manufacturing, safety, and the next round of pharmaceutical breakthroughs.
1-Methylpiperazine belongs to the piperazine family, which shows up a lot in medicinal chemistry. Its formula—five carbons, twelve hydrogens, and two nitrogens—points directly to how the molecule gets used. That single methyl group attached to the piperazine ring changes its behavior completely compared to the plain version. Medicinal chemists see an opportunity here: change one part of the ring, and suddenly you might get a compound with better solubility or a safer toxicity profile. For decades, this approach drove drug innovation. Without understanding or trusting the formula, progress grinds to a halt.
Anyone working in a lab learns the importance of formulas the hard way. If a technician weighs the wrong amount because of a miscalculation, or the formula’s written out wrong in a protocol, you can end up with a useless product. When someone’s testing a sample or calibrating equipment, accuracy matters—down to the last hydrogen atom. My old research group once ordered a methylpiperazine isomer by mistake due to sloppy notation, setting our experiments back weeks. Lives in the pharmaceutical industry revolve around these tiny details. Getting formulas right means keeping toxic intermediates out of the final pill, protecting workers, and cutting down waste.
The formula C5H12N2 doesn’t just guide synthetic routes—it shapes handling rules. Two nitrogen atoms make it basic, influencing how it acts in water and interacts with other ingredients. These properties sway everything from storage regulations to shipping paperwork. Chemical companies invest millions in safety protocols because even small molecules like 1-Methylpiperazine can be volatile in the wrong conditions. The formula lays out the foundation for hazard assessments and emergency response plans.
Many newcomers ask why they must memorize such formulas. Direct exposure teaches the answer. Misreading a blueprint causes costly errors; knowing formulas by heart gives a team the power to troubleshoot on the spot. In my own teaching experience, repeated drills on small molecules gave new hires the confidence to flag problems before a batch failed downstream. Skilled chemists don’t guess their way around; they work with precision rooted in knowledge of building blocks like C5H12N2.
A future with safer labs starts with stronger education. Mistakes around chemical formulas sometimes lead to injuries. Employers can mitigate risk through clear labeling practices, better software tools, and encouraging a culture where no one feels embarrassed to double-check a formula on the job. Digital databases now make it easier for everyone to access the latest structural info, but checking against reputable sources remains key. Tracing the journey from a single formula to the final medicine gives people a real reason to care.
Working in a lab teaches you that chemical safety isn’t something to take lightly. Some people might think common solvents are safe as long as caps stay on tight, but not all chemicals play by those rules. 1-Methylpiperazine doesn’t always make headlines, yet mishandling can cause financial losses, ruined research, and safety hazards.
1-Methylpiperazine is a colorless liquid with a strong fishy odor. Lab folks quickly recognize that smell—definitely not something to welcome in break rooms or storage closets. Its flash point sits at about 39°C (102°F). That means ordinary room temperatures on a hot day bring this chemical closer to danger if no precautions exist. This volatility isn’t just a paperwork issue. Reports of minor fires remind us why solid storage protocols matter.
Temperature plays the biggest role. If you stick 1-Methylpiperazine next to a sunny window or leave it in a hot warehouse, you’re asking for trouble. Never storing it near heat sources or in areas with direct sunlight makes a big difference. In my earlier lab days, someone once placed amines near a radiator and we lost half a week sorting out the mess.
A cool, dry place wins every time. Most labs keep this chemical at temperatures below 25°C (77°F) to avoid vapor buildup or accidental ignition. Cold rooms—provided the chemical is well-sealed and labeled—offer another layer of safety when ambient temperatures can’t be guaranteed.
Good ventilation isn’t an extra; it’s essential. I’ve seen a closed cabinet loaded with strong amines turn into a low-level hazard because vapors got trapped. Fume hoods or flame-proof cabinets with venting really do cut risks. Don’t stash 1-Methylpiperazine with acid bottles or oxidizers, either. Reactivity doesn’t always need much to go wrong.
Always use tightly-sealed containers—glass or compatible plastics. Having a secondary containment tray below all containers will catch leaks before they soak shelves or floors. That step helped us avoid a big cleanup more than once. Labels must be clear and include hazard information, not just abbreviations. Relying on memory is a shortcut that backfires.
Moisture messes with the purity of amines. 1-Methylpiperazine can absorb water from the air, so keeping lids on and working quickly pays off. Using desiccants in storage cabinets slows down this problem.
Routine inspection matters. Check containers every few months for leaks or crusty buildup around the lids. That kind of damage signals time for replacement before exposure gets worse or cross-contamination happens.
Limit who gets near your chemical inventory. Only those who understand its risks should access chemicals like 1-Methylpiperazine. Posting storage instructions and safety sheets near storage shelves reduces confusion when new team members join.
Have spill kits and firefighting equipment nearby, not locked away in some distant supply closet. The best teams I’ve seen run through emergency drills until grabbing the right gear becomes second nature.
Authorities like OSHA, the CDC, and chemical suppliers share reliable guidelines for storage. Following published safety data sheet (SDS) advice pays off more than risky shortcuts based on habit or hearsay. That approach keeps people and research budgets safer every year.
Anyone with a background in pharmaceuticals or industrial chemicals has come across building block ingredients like 1-Methylpiperazine. This compound sits on the ingredient lists of various medicines and agrochemicals. It isn’t a household name, but it shapes more products and processes than many realize. Chemists rely on it for synthesis or as part of specialized formulations. The reason for checking bulk availability isn’t just curiosity—it’s about ensuring that supply matches demand so research and large-scale manufacturing aren’t held back by bottlenecks.
Anyone who’s had to track down raw materials for a lab or production line knows the pain points: supply shortages, inconsistent quality, fluctuating prices, and complex regulation. 1-Methylpiperazine sometimes falls into this puzzle. Not every supplier can handle or ship large amounts. The world supply web is stretched across continents. Major chemical suppliers in China, India, and Europe list bulk quantities, but the journey from factory to buyer involves paperwork, satisfying safety laws, and quality checks—a process that eats up both time and resources.
Safety is another piece of the story. Transporting amines in large volumes requires tight control. Leaks trigger environmental hazards. Regulations aren’t just red tape—they exist because someone, somewhere, skipped a step, and the fallout cost real lives and money.
From my experience, sourcing 1-Methylpiperazine in bulk isn’t out of reach, but it’s not as simple as buying baking soda at the corner market. Some of the biggest chemical vendors in the world stock it, typically ranging from small bottles for lab scale up to drums suitable for factory runs. Purchases in the hundreds or thousands of kilos require more than a click. Buyers and sellers sign contracts, lock in prices, and often undergo background checks due to regulations aimed at monitoring precursor chemicals.
Many middle-market buyers—smaller manufacturers and contract research outfits—hit an odd spot. They’re not big enough to command discounts or custom lots from producers, but their demand outpaces what typical lab suppliers offer. This is where regional brokers or specialty distributors step in, often acting as the go-between. While this helps, it nudges up the cost compared to direct-from-factory deals.
Better supply pipelines start with transparency. Buyers benefit when producers openly share data about inventories, production schedules, and compliance documents. Some larger chemical firms maintain online portals that track not just availability, but order progress and regulatory paperwork, making life easier for procurement teams.
Regulatory knowledge keeps the wheels turning. Startups and small firms sometimes run into roadblocks simply because they underestimate the paperwork. Many regional chemical trade groups provide training on proper sourcing and handling. Their guidance saves headaches and keeps business moving.
Sustainable access to chemicals like 1-Methylpiperazine matters far beyond research. It holds up entire pipelines for new medicines, crop treatments, and specialty materials. Strong relationships with reliable suppliers, paired with staying in tune with market and regulatory changes, make all the difference. Sourcing teams who stay proactive—not just reactive—help ensure the wheels of innovation never slow down just because a shipment got stuck en route.
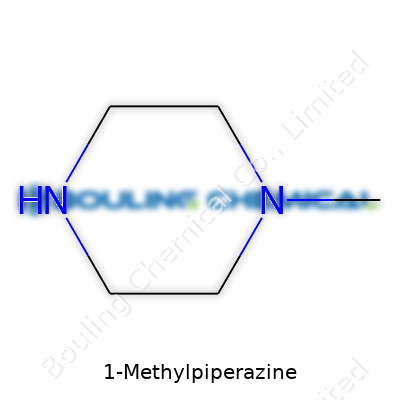
| Names | |
| Preferred IUPAC name | 1-Methylpiperazine |
| Other names |
N-Methylpiperazine Piperazine, 1-methyl- 1-Methyl-1,4-diazacyclohexane N-Methyl-1,4-piperazine N-Methyl-hexahydropyrazine |
| Pronunciation | /ˈwʌnˈmɛθ.ɪl.paɪˈpɛr.əˌziːn/ |
| Identifiers | |
| CAS Number | 109-01-3 |
| Beilstein Reference | 1081659 |
| ChEBI | CHEBI:35293 |
| ChEMBL | CHEMBL924 |
| ChemSpider | 7279 |
| DrugBank | DB07756 |
| ECHA InfoCard | 100.017.849 |
| EC Number | 202-585-2 |
| Gmelin Reference | 82996 |
| KEGG | C06298 |
| MeSH | D010868 |
| PubChem CID | 31237 |
| RTECS number | TZ9625000 |
| UNII | RR6CB8Y77E |
| UN number | UN2734 |
| Properties | |
| Chemical formula | C5H12N2 |
| Molar mass | 100.18 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | Ammonia-like |
| Density | 0.911 g/mL |
| Solubility in water | miscible |
| log P | -0.30 |
| Vapor pressure | 0.5 mmHg (at 25 °C) |
| Acidity (pKa) | 9.8 |
| Basicity (pKb) | 4.87 |
| Magnetic susceptibility (χ) | -6.7×10⁻⁹ |
| Refractive index (nD) | 1.457 |
| Viscosity | 0.773 cP (25°C) |
| Dipole moment | 2.32 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 308.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -24.0 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3789.6 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS05, GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H314, H319, H335 |
| Precautionary statements | P264, P280, P302+P352, P305+P351+P338, P337+P313, P332+P313, P362+P364 |
| NFPA 704 (fire diamond) | 2-3-0 |
| Flash point | 74°C |
| Autoignition temperature | 250 °C |
| Explosive limits | 3.4% - 22.5% |
| Lethal dose or concentration | LD₅₀ (oral, rat): 2840 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 2,140 mg/kg |
| NIOSH | RN785 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 ppm |
| IDLH (Immediate danger) | 800 ppm |
| Related compounds | |
| Related compounds |
1,4-Dimethylpiperazine 2-Methylpiperazine Piperazine N-Methylpiperazine |