Understanding how 1-Methyl-4-Piperidyl Diphenylglycolate made its way from lab benches into specialized applications starts with its roots in mid-20th century organic chemistry. Back then, the pharmacological community was hungry for effective anticholinergic compounds. This molecule, part of the broader piperidyl glycolate family, came into focus when chemists sought agents that could block muscarinic acetylcholine receptors. They weren’t chasing obscurity—a surge of interest in spasmolytics and compounds capable of relieving smooth muscle contraction shaped much of medicinal research. Initial patents and publications surfaced in Europe, especially in Germany, where industry giants collaborating with academia tested derivatives in animal models and charted out their structures through labor-intensive methods, much before NMR and mass spec ruled the scene. Years of incremental tinkering with the parent scaffold gave rise to a class of compounds, each step yielding better insights into therapeutic promise and hazards.
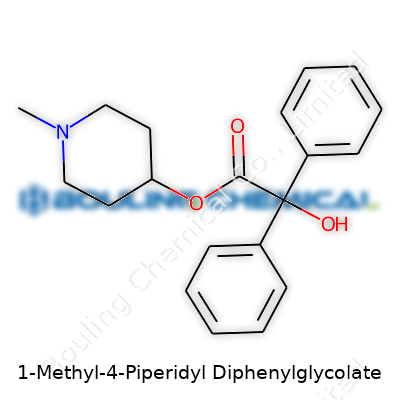
Walking into a fine chemicals catalog today, you find 1-Methyl-4-Piperidyl Diphenylglycolate among other sophisticated anticholinergics. Its chemical structure holds a piperidine ring coupled to a glycolate backbone, flanked by two big phenyl rings—a layout designed to wedge itself into biological receptors snugly. Companies typically offer it as a white to off-white crystalline solid. The commercial product often comes with high purity, as research and formulation demands leave little room for uncertainty about contaminants. Downstream users, from university research labs to pharmaceutical development teams, rely on its consistency for reliable results in pharmacological studies.
On the physical side, you’re looking at a melting point just above that of everyday compounds, landing it in the ballpark of therapeutic alkaloids. It's soluble in polar organic solvents like ethanol, chloroform, and dimethyl sulfoxide. This solubility makes it easier to prep for assays and derivatization. There’s a noticeable faint aroma when working at the bench, a reminder of its piperidyl core. Chemically, the molecule is fairly stable under dry and cool storage conditions, but like many esters, it doesn't do well in the presence of strong acids or bases, where hydrolysis can chip away at its glycolate group. Its two phenyl groups lend a degree of lipophilicity, affecting both its passage through biological membranes and extraction efficiency during purification.
Every bottle tells a story through its label. The best suppliers don’t skimp on specifying the content, lot number, purity (often above 98%), and storage recommendations—typically room temperature away from bright light. Some also provide spectral data—proton NMR, IR, and a certificate of analysis. This detail isn’t only about regulatory compliance; researchers trust that what’s inside the bottle matches the published standards. Molecular weight clocks in at around 363 g/mol, with a chemical formula of C21H25NO3. Shipment falls under standard hazardous goods provisions due to its potential toxicity. Since the molecule is a research chemical, not for use in humans or veterinary medicine, most labeling makes that perfectly clear.
Organic chemists bring 1-Methyl-4-Piperidyl Diphenylglycolate to life using a multistep approach. The main event involves coupling 1-methyl-4-piperidinol with diphenylglycolic acid using a dehydrating agent like dicyclohexylcarbodiimide (DCC) or EDC. Some tweak the method, using acid chlorides or anhydrides to boost yield. Every step—from the drying of solvents to recrystallization—demands careful control to chase off water and prevent unwanted side reactions. Yields vary with the batch size and reaction finesse, but skilled hands regularly push past 80%. Purification almost always relies on silica-gel column chromatography, followed by vacuum drying to remove any stubborn traces of solvents.
The glycolate ester group begs for exploration. Hydrolysis splits off the phenylglycolic acid or piperidinol in the presence of acid, base, or even enzymes, which is handy for probing metabolic fate or designing prodrugs. The piperidyl nitrogen allows quaternization or alkylation to tune its pharmacodynamic profile. Substituting different groups onto the phenyl rings has generated close analogues—each tweak offering a shot at reduced side effects or longer duration of action in vivo. Chemists have even tried introducing electron-withdrawing or electron-donating substituents, revealing how small changes ripple through binding affinities at the molecular target.
A trip through the chemical literature uncovers aliases like 1-Methyl-4-piperidyl α,α-diphenylglycolate and alpha,alpha-Diphenylglycolic acid 1-methyl-4-piperidyl ester. Pharmacopeial references sometimes call it methylpiperidyl diphenylglycolate. Those in the industry might shorten it to MDG for quick reference, though the full name often appears in regulatory documents and research publications to avoid ambiguity. It occasionally crops up in lists of inactive ingredients or in catalogs of chemical intermediates for medicinal chemistry programs.
Working with 1-Methyl-4-Piperidyl Diphenylglycolate calls for standard laboratory precautions. Gloves, goggles, and lab coats form the baseline. Spills shouldn’t be shrugged off; the compound's absorption through the skin can’t be discounted, especially since anticholinergic side effects come on quick and strong. Fume hoods help keep exposure under control, especially during weighing and transfer. Disposal runs through organic waste streams, handled by professionals familiar with hazardous materials. Data sheets warn against ingestion, inhalation, or skin contact, and anyone who’s handled the compound learns to respect its power as a pharmacological agent outside intended research settings.
Pharmacologists cut their teeth on 1-Methyl-4-Piperidyl Diphenylglycolate as they trace the threads of muscarinic receptor antagonism. It often serves as a reference compound in bioassays. Researchers eager to test new analogues use it as a benchmark. Beyond traditional pharma settings, some academic teams explore its structure-activity relationships to design new tools for neuroscience. Toxicology studies depend on its well-characterized effects to set baselines. The compound also surfaces in studies examining blood-brain barrier penetration for central nervous system drug development. Its inclusion in research projects reflects both its established safety profile (in controlled settings) and its potency as an investigative tool.
Every major R&D push in anticholinergic drug discovery has a footprint from related piperidyl glycolates, and this molecule stands out for its balance of potency and manageability. Teams working to map muscarinic receptor subtypes and functions pull it from the shelf to inhibit specific pathways and measure corresponding outcomes. Modern computational chemists apply docking studies to predict how analogues of this molecule might behave, peering into the future of drug design by starting with old workhorse scaffolds. This iterative process, connecting molecular tweaks to physiological changes, keeps the compound relevant in academic and startup circles alike. Emerging research also uses the compound to test new delivery platforms, whether sustained-release formulations or targeted nanoparticles, to see if established actives can perform even better with a technological boost.
Every compound worth studying demands attention to toxicity. Early preclinical work flagged classic signs of anticholinergic poisoning: dry mouth, increased heart rate, blurred vision, sometimes even central nervous effects at higher doses. Rodent studies revealed clear dose-response relationships, and researchers quickly learned safety margins. That said, no one in their right mind would use this compound therapeutically outside the most controlled clinical research settings. Ongoing studies keep looking for ways to moderate these effects or stop them outright with antidotes. Researchers track not just anecdotal reports but gather mountains of data on LD50, organ specificity, and long-term exposure. This layer of caution ensures that interest in structural analogues or formulations never gets far ahead of basic safety understanding.
Looking ahead, the continued pull of muscarinic antagonists in treating neurological and gastrointestinal disorders keeps interest in 1-Methyl-4-Piperidyl Diphenylglycolate alive. Advances in receptor subtype specificity mean chemists revisit old scaffolds, trying to eke out new selectivity with smart modifications. Technology has made high-throughput screening and in silico modeling more accessible, so the molecule serves as both a template for new compounds and a comparator for efficacy and toxicity studies. As regulatory standards tighten, companies and research labs alike need to demonstrate crystal-clear safety profiles and batch reproducibility. Any breakthroughs in tissue-targeted delivery or antidote development could rekindle clinical interest in these structures. Its future isn’t set in stone, but it remains a touchstone for anybody digging deep into the interface of organic chemistry and pharmacology.
1-Methyl-4-Piperidyl Diphenylglycolate doesn’t pop up in everyday conversation, but it has a big job in the world of pharmacology. At its core, this chemical acts as an anticholinergic compound. The main action involves blocking acetylcholine, the messenger that nerve cells use to talk with each other and muscles. In practical terms, by keeping those signals from connecting, the compound calms spasms in muscles and relaxes smooth muscle tissue.
I’ve seen patients struggle with muscle cramps that disrupt everything from sleep to daily routines. Medicines working along anticholinergic lines often bring real relief. That’s where 1-Methyl-4-Piperidyl Diphenylglycolate fits into the picture. As an active ingredient or as a research precursor, it helps researchers and clinicians handle tough cases linked to muscle spasm or overactive nerves.
Doctors treating irritable bowel syndrome or peptic ulcer pain know the importance of antispasmodic drugs. This compound steps in to quiet uneasy muscles in the gut, making it easier to treat discomfort without turning to heavy-duty painkillers right away. While no single drug solves all gut issues, targeting the nerves that drive gut cramping often spells the difference between suffering and relief. An old mentor of mine would always say, “Spastic colon meets its match in a well-chosen anticholinergic.” He wasn’t wrong—these treatments have stayed relevant for decades.
Some compounds with similar structures even help with motion sickness, overactive bladder, and muscle rigidity in neurological problems like Parkinson’s disease. By slowing down involuntary muscle movement, these drugs can restore dignity to folks who have lost it to illness. I’ve worked with patients using these medications who can go out in public again, no longer afraid of unpredictable muscle spasms.
Despite the benefits, these treatments don’t come without baggage. Anticholinergic medicines often cause dry mouth, blurred vision, confusion in older adults, and sometimes trouble urinating. Elderly folks walk a particular tightrope here—cognitive issues can sneak up, and that’s something I’ve noticed working in geriatric clinics. A family member experienced cognitive fog after taking a similar muscle relaxant, leading our physician to re-evaluate the prescription. Close monitoring and starting with the lowest effective dose matter more than any marketing pitch.
Smart prescribing can keep problems in check. Regular check-ins between patient and provider spot side effects before they grow. There’s growing interest in designing new medications that offer muscle relaxation or relief from nerve irritation without clogging up the rest of the system. Research into targeted delivery—think drugs that home in on GI tissue instead of spreading everywhere—sets a higher bar for safety down the line. I believe every improvement in drug design comes from listening to the real stories of patients dealing with both relief and unwanted effects.
Regulation matters too. In some regions, researchers use 1-Methyl-4-Piperidyl Diphenylglycolate to develop new therapies, with strict oversight. As with most medicines affecting neurochemical signals, access and use need a careful partnership between scientists, pharmacists, and clinicians.
Anticholinergic chemistry stands as both a boon and a warning. The relief these medicines provide sits next to a set of side effects that can drag down quality of life if unchecked. Patients deserve solutions built around actual needs, not just chemical novelty. That’s what keeps both science and medicine honest.
Talk of 1-Methyl-4-Piperidyl Diphenylglycolate rarely comes up unless chemistry textbooks form your bedtime reading or you work in specialized pharmacology. Not a household name, this compound sits in a family of anticholinergic agents. Its framework echoes compounds with powerful effects on the nervous system. The long, clunky chemical name might seem intimidating, but for anyone wondering about dosage, real-life experience and scientific care go hand in hand.
Science has a firm boundary here: there’s a massive difference between research chemicals and substances approved for human use. Before even touching a dosage chart, it pays to remember that 1-Methyl-4-Piperidyl Diphenylglycolate has not seen much clinical discussion. This isn’t a drug you can pick up at a pharmacy, and it doesn’t headline any guidelines published by the FDA or other regulatory bodies. In fact, traceable research tends to mention its use in animal studies or in chemical research related to nerve agent antidotes or anti-spasmodics, rather than regular medical practice.
Personal stories in the toxicology world show the danger of treating unregulated compounds like over-the-counter tablets. Misjudged dosages risk a laundry list of side effects—dry mouth, blurred vision, rapid heartbeat, confusion, even delirium. These effects come from the way anticholinergics block acetylcholine, a neurotransmitter crucial for muscle and brain function. Too much blockade scrambles both body and mind. In the absence of official dosage recommendations, untrained use drifts toward risk, not remedy.
Rather than reaching for numbers pulled from guesswork or fragmented animal studies, transparency needs center stage. A search through scientific literature often lands at studies with rodents, not people. In the field, experts rely on a bedrock of tested clinical trials and published data before ever tallying up milligrams for humans. This compound’s pathway through the regulatory system remains unmarked. For doctors and scientists, that means sticking to tested, approved medications with a known dosing range and documented safety profiles. That principle guards against the unknowns of a chemical whose effects in people still lack thorough investigation.
Building a culture that depends on solid science rather than curiosity-driven experimentation keeps people safer. In my years sifting through both published studies and real-world medical cases, the lesson holds true: detailed peer-reviewed evidence shapes every prescription and treatment guideline worth following. If a promising chemical catches interest, the right solution starts with formal research—double-blind trials, peer review, transparent adverse effect reporting. Until then, any so-called recommended dosage veers into dangerous territory.
People with medical questions need trustworthy answers. For now, the conversation around 1-Methyl-4-Piperidyl Diphenylglycolate doesn't lead to a neat dosage range, but to a clear message: unapproved compounds belong in research labs, not medicine cabinets. Instead, working with health professionals and using treatments backed by clear, public evidence keeps risks in check and trust intact. If curiosity sparks questions, the call goes out for careful science, not shortcuts.
Questions about drug safety have always had a special place in pharmacy and healthcare. People put a lot of trust in doctors and pharmacists. It pays to have honest answers on what a chemical like 1-Methyl-4-Piperidyl Diphenylglycolate can do to the body. This synthetic compound has roots in the field of anticholinergic drugs. It works by blocking the neurotransmitter acetylcholine in the nervous system. These drugs have been useful in many ways, sometimes prescribed for motion sickness or muscle spasms. But every medicine, helpful as it might be, brings along some risk.
People who have taken anticholinergic-type drugs often describe dry mouth or feeling as if swallowing is harder than usual. This sensation lasts longer with repeated doses. Blurred vision or trouble focusing on printed words may follow. I have heard people speak about feeling unsteady, almost lightheaded. Changes in mood can sneak up, including swings toward irritability. Older patients mention confusion that can get mixed up with forgetfulness from age alone. Constipation and difficulty urinating are other sides few enjoy discussing, but they crop up with these kinds of chemicals.
Studies back up what people describe. Research in clinical settings shows that anticholinergic drugs, including 1-Methyl-4-Piperidyl Diphenylglycolate, disrupt normal nerve signals across many organ systems. The body's glands make less spit and sweat. The gut moves slower, making bowel movements harder to pass. The eyes adjust slower to changing light. Heart rhythm can speed up, sometimes putting patients at risk of palpitations. Safety studies point to the risk of toxicity if someone takes too much, with restlessness, confusion, and, in severe cases, hallucinations.
Long-term or high-dose use does not only raise the likelihood of side effects. It can increase the risk of lasting problems. For older patients, anticholinergic burden builds up quietly. Evidence links these drugs with greater risk of dementia-related symptoms when used repeatedly over many months or years. For example, the British Medical Journal once published research that connected higher cumulative exposure to strong anticholinergics with increased risk of cognitive decline.
Doctors and patients need straightforward conversations about side effects and what to watch out for. Careful medication reviews make a difference, especially for people taking more than one drug. Lowering the dose or switching to alternatives with fewer anticholinergic properties often helps. Staying hydrated and eating more fiber supports the digestive system. Automated systems in hospitals now flag dangerous drug combinations, helping reduce mistakes.
Patients should be encouraged to bring up symptoms, even those that seem minor. No one should stick with new confusion or trouble seeing without telling their doctor or pharmacist. Good healthcare draws on science, yes, but also on honest voices from everyday life. Listening to side effect reports has shaped safer prescribing for years.
1-Methyl-4-Piperidyl Diphenylglycolate doesn’t roll off the tongue easily, but it pops up in clinical and pharmaceutical discussions. Commonly, this compound has strong ties to research in neurology and pharmacology. For those outside of a chemistry lab, the most familiar uses come as an ingredient in treatments for certain nervous system disorders.
Medicine cabinets shouldn’t look like a chemistry classroom. There’s a reason some compounds stay behind the pharmacy counter. Safety ranks as a driving force. 1-Methyl-4-Piperidyl Diphenylglycolate works on the nervous system. Without medical supervision, even a small mistake in dosage could cause confusion, hallucinations, or rapid heartbeat. Support for these restrictions comes from studies showing that anticholinergic agents, such as this compound, carry real risks, especially among older adults or those with certain chronic illnesses. According to the Food and Drug Administration in the United States and the European Medicines Agency, medications of this nature stay in a controlled class for patient protection.
Walk into a pharmacy in most countries, and pharmacists won’t hand over powerful nervous system agents without seeing a note from your doctor. A few years ago, a mix-up with a similar compound in a community practice led to a child suffering an emergency room visit for agitation and dizziness. The medical team traced it back to accidental misuse. This isn’t a scare story; just a glimpse into realities that come up far more often than anyone wants to admit.
Any drug that acts on the brain or heart tends to demand a physician’s oversight. According to the World Health Organization, access to prescription drugs remains one of the key methods to prevent drug abuse and accidental overdose. Rules around prescriptions protect not only the person seeking relief but also help limit misuse and diversion into non-medical channels.
People argue that easier access to medicines saves time and puts the power in patients’ hands. In many cases, like cold remedies or allergy pills, this makes sense. But substances that can cause severe psychiatric or cardiovascular reactions cross a line. Making them available without a prescription could open the door for dangerous experimentation. My time volunteering at a public health clinic taught me that families can face tragedy not just from a lack of medication, but just as often from access to the wrong drugs.
Calls to relax access to prescription medications come up in debates, especially as online pharmacies grow. Improved patient education, electronic prescription tracking, and stronger ties between patients and healthcare teams all play a role in safer medicine use. Shifting from a one-size-fits-all approach to better personalized risk assessments could solve some access challenges, but responsible gatekeeping still matters most for substances like 1-Methyl-4-Piperidyl Diphenylglycolate.
Medicine isn’t just about formulas and bottles—it’s about keeping people safe. The verdict on 1-Methyl-4-Piperidyl Diphenylglycolate is clear for a reason. Without proper oversight, this compound’s power could do more harm than good. Prescription requirements exist to protect health, based on decades of evidence and the stories of real people affected by misuse. Convenience never outweighs safety where serious risks stand in the way.
Every time I help out in the lab, the top worry leans toward chemical safety, not just during experiments, but also before and after. 1-Methyl-4-Piperidyl Diphenylglycolate isn’t something you want sitting around in a haphazard fashion. This compound often pops up in pharmaceutical research, and from my own work with similar chemicals, I can say that the decisions made about storage often spell the difference between safety and disaster.
From published safety data, 1-Methyl-4-Piperidyl Diphenylglycolate carries health risks if inhaled or if it contacts the skin. Volatility isn’t its main trait, but exposure over time degrades the product and increases risks for everyone in the room. Proper storage preserves both the chemical’s integrity and the safety of colleagues. A little care goes a long way—something that doesn’t always get enough attention during a busy workday.
Temperature stands out as a critical factor. Every chemical cabinet in my lab stays checked for working thermometers because small variations add up. Most safety sheets call for cool, dry storage, away from sources of heat. In my experience, shelf life takes a hit any time vials get left on open benches, so it’s better to keep this compound in a well-ventilated storeroom set to a controlled temperature.
Light exposure matters just as much. Similar compounds quickly lose their punch when left in direct sunlight or under harsh fluorescent lighting. I encourage colleagues to use amber glass containers or opaque bottles, and I label boxes well so no one accidentally pulls the wrong bottle in poor lighting.
Moisture always finds a way in if you give it a chance. Humid air speeds up chemical breakdown, so I use desiccants inside storage cabinets whenever possible and double-check that rubber stoppers or screw caps seal tightly. Many times I’ve seen labels curling from moisture, a red flag that storage needs reevaluation.
Exposure to air can trigger slow oxidation, even with chemicals that don’t make it obvious on first glance. Testing vials and storage tubes for tight seals becomes routine in my team’s safety walk-throughs.
Some labs keep a single key for all controlled substances. I’ve seen arguments break out when someone misplaces a vial or forgets to sign out a chemical. Logging each bottle’s movement, using a digital system or a paper log, eliminates confusion and lowers the odds of an accident. If there’s ever a spill or a missing bottle, having clear records saves valuable time and stress.
Accidents never announce themselves. During orientations, I highlight exactly where to find spill kits, eye wash stations, and safety showers. Anyone who works near 1-Methyl-4-Piperidyl Diphenylglycolate needs to know the protocol for exposures and what to do if a container cracks or leaks. Running drills twice a year ensures everyone reacts fast and cuts panic.
Safety culture shows up in the way lab teams treat the most basic steps. Protecting chemicals from light, humidity, and heat becomes second nature with training and reminders around the lab space. Chemical storage isn't just about compliance—it’s about looking out for each other. The more consistently these habits stick, the safer every project turns out, whether handling 1-Methyl-4-Piperidyl Diphenylglycolate or anything else that comes across the bench.
| Names | |
| Preferred IUPAC name | 1-methyl-4-phenyl-1-piperidinyl 2,2-diphenyl-2-hydroxyacetate |
| Other names |
Euridine Srilaren Mepenzolate |
| Pronunciation | /waɪˈmɛθɪl fɔːr paɪˈpɛrɪdɪl daɪˈfɛnɪl ɡlaɪˈkəʊleɪt/ |
| Identifiers | |
| CAS Number | [3614-47-9] |
| 3D model (JSmol) | `/ajax.php?modelid=05678&format=jsmol` |
| Beilstein Reference | 3538165 |
| ChEBI | CHEBI:90680 |
| ChEMBL | CHEMBL2105981 |
| ChemSpider | 21476880 |
| DrugBank | DB08908 |
| ECHA InfoCard | ECHA InfoCard: 100.073.980 |
| Gmelin Reference | 107188 |
| KEGG | C11722 |
| MeSH | Dyl MeSH: D010869 |
| PubChem CID | 64743 |
| RTECS number | GN8575000 |
| UNII | TG2M9WT13E |
| UN number | UN3276 |
| CompTox Dashboard (EPA) | DTXSID4021577 |
| Properties | |
| Chemical formula | C20H25NO3 |
| Molar mass | 363.47 g/mol |
| Appearance | White solid |
| Odor | Odorless |
| Density | 1.14 g/cm³ |
| Solubility in water | Insoluble |
| log P | 1.83 |
| Vapor pressure | 5.16E-6 mmHg at 25°C |
| Acidity (pKa) | pKa = 8.6 |
| Basicity (pKb) | 3.57 |
| Magnetic susceptibility (χ) | -73.2×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.591 |
| Dipole moment | 3.73 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 354.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -285.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1075.1 kJ/mol |
| Pharmacology | |
| ATC code | A03AB03 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P210, P261, P264, P280, P301+P312, P305+P351+P338, P337+P313, P405, P501 |
| NFPA 704 (fire diamond) | Health: 2, Flammability: 1, Instability: 0, Special: – **String:** `"2-1-0"` |
| Flash point | Flash point: 153.2°C |
| Autoignition temperature | 400°C |
| Lethal dose or concentration | LD50 oral rat 660mg/kg |
| LD50 (median dose) | LD50 (median dose): 517 mg/kg (oral, rat) |
| NIOSH | DD6382000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 1-Methyl-4-Piperidyl Diphenylglycolate: Not established |
| REL (Recommended) | 10 mg/m³ |
| Related compounds | |
| Related compounds |
3-Quinuclidinyl benzilate N-Methyl-3-piperidyl benzilate Penehyclidine Dihepyridine Benactyzine Diphenhydramine |