Chemistry never stands still. Back in the mid-twentieth century, researchers working through libraries of piperidine derivatives noticed small changes to these classic ring structures could spark big differences in reactivity. 1-Methyl-4-Chloropiperidine arrived on the scene as chemists explored new synthetic intermediates with the hope of simplifying routes to pharmaceuticals. Forward-looking labs in Europe and the United States dug deeper, logging findings about how a methyl group at the nitrogen and a chlorine at the fourth carbon shifted its chemical personality. University researchers and pharmaceutical companies in the 1960s and ’70s added it to libraries of building blocks, drawing on work published in journals like the Journal of Medicinal Chemistry. These notes inspired others to tweak and probe it, pushing its synthesis and applications into modern organic chemistry.
Scientists dealing with this molecule handle a colorless to pale yellow liquid with an unmistakable, pungent odor—harsh in a way that sticks around, even after washing glassware. Its formula, C6H12ClN, gives away the small but important decorations on the piperidine backbone. Specialty chemical companies supply it to pharmaceuticals, agrochemical firms, and research groups, usually in small to medium-sized bottles. In the lab, it rarely gets the spotlight, but experienced chemists recognize its value in making more complex molecules, especially for pharmaceutical lead compounds or test reactions that involve nitrogen chemistry.
You pick up a bottle of 1-Methyl-4-Chloropiperidine and quickly notice its low boiling point—around 155°C—and its high vapor pressure at room temperature, making it both handy for distillation and easy to lose through evaporation. Its density lines up near the expected mark for medium-weight amines, and its miscibility tells you it likes most organic solvents but gives water a wide berth. With a strong basic center at the nitrogen and an electron-withdrawing chlorine, the molecule sometimes surprises folks trying to predict its behavior in coupling reactions. Its color and consistency make spills obvious, yet cleanup isn’t as straightforward as you might wish—the odor always gives away its presence. Storage in cool, dry conditions with good ventilation, away from open flames, keeps things safe and stable.
Safety labeling on 1-Methyl-4-Chloropiperidine shouts danger, not caution. Labels display hazard pictograms for flammability, acute toxicity, and environmental harm. Industrial bottles list purity, typically above 98% for research use, batch number, CAS number (104-89-2), and manufacturer. Documentation details not only chemical identity but also strict transport regulations. Global organizations, from OSHA to REACH, require clear signal words like "Danger" and list out first aid and storage instructions, all designed to protect those who choose to work with it.
Labs make 1-Methyl-4-Chloropiperidine through classic alkylation and halogenation reactions. A common path begins with piperidine or N-methylpiperidine, followed by chlorination at the right spot—often via a mixture of chlorinating agents and solvents that tolerate basic conditions. Sometimes, chemists swap protecting groups mid-reaction or control temperatures to avoid side products. Making a pure batch demands careful distillation or chromatography to coax the target molecule away from similar cousins. It’s not a process for beginners, so chemical suppliers scale up under strictly controlled conditions with exhaust air capture for both environmental and worker safety.
This molecule’s real fame lies in what you can turn it into. That methyl group at nitrogen blocks a whole class of acylation reactions, forcing chemists to try other routes if they want to build larger molecules. The chlorine atom attracts nucleophiles, letting researchers push through substitutions that swap it for nearly anything: amines, thiols, or alkoxides depending on the solvent and temperature. Sometimes, folks leverage both sides, building out spiro or fused ring structures for experimental drugs where precise shape matters. The array of possibilities keeps inventive chemists coming back, using it as a base unit to test new methods or hunt down promising pharmacophores.
1-Methyl-4-Chloropiperidine pops up in catalogs and journals under several names. Some call it N-Methyl-4-chloropiperidine, while others shorthand it as 4-Chloro-1-methylpiperidine or 1-Methyl-4-chloro-hexahydropyridine. Its CAS number, 104-89-2, offers a universal identifier that helps avoid mix-ups. Different suppliers sometimes give their own twists—adding company abbreviations or referencing pharmaceutical standards—but the core name stays rooted in the IUPAC convention. This clear identification cuts down on errors, especially during scale-up or cross-border shipment.
Anyone handling this chemical wears gloves and goggles—not negotiable. Its toxicity and volatility signal real risk if inhaled or spilled on skin. Labs set up fume hoods, mandate double-checks of seals, and keep spill kits nearby. Folks know not to eat or drink in work areas. For industrial setups, strict ventilation draws vapors away from workers, and monitoring equipment watches for leaks. Regulatory reports cite risk of neurological symptoms or respiratory distress on exposure. Companies train workers in emergency procedures—eyewash stations, ventilation checks—because one mistake can mean serious consequences. Safe disposal follows hazardous waste regulations: sealed, labeled containers and clear documentation for incineration or other treatment.
Specialty chemical producers pitch 1-Methyl-4-Chloropiperidine to pharmaceutical research, especially where folks need a springboard to synthesize central nervous system agents or structurally novel antihistamines. Agrochemical designers reach for it when they’re searching for new routes to active ingredients that fight pests or fungi. Analytical chemists use it as an intermediate to test new analytical techniques. In teaching labs, skilled instructors sometimes show off its reactivity to challenge students, though only in tightly controlled settings. Once in a while, it finds its way into flavor chemistry research—though its overpowering odor generally rules it out from direct food industry work.
Recent research highlights the molecule’s versatility as a test case in computational chemistry: its structural quirks make for hard but rewarding modeling projects. Ongoing studies use it as a probe molecule or a model for nucleophilic substitution at hindered aliphatic carbons. Medicinal chemists push its modifications to explore new scaffolds in the hunt for non-opioid painkillers and CNS modulators. Green chemistry groups experiment with catalytic routes or cleaner halogenation steps, trying to trim the environmental toll. Chemical engineers look at continuous flow techniques to boost safety and purity on larger scales. Teams compare it with newer piperidine analogs, scoring or ranking derivatives for future development.
Most information on toxicity comes from animal studies and workplace exposure reports. Inhalation leads to dizziness, headache, or worse, and skin contact can cause burns or systemic effects. Chronic exposure links to nerve damage, though data lags behind newer compounds. Animal tests and in vitro studies probe for mutagenic or carcinogenic potential, but the evidence stays patchy. Regulatory agencies keep the permitted airborne levels low, and careful tracking of occupational health incidents leads to ongoing tightening of exposure limits. Researchers continue to run long-term tests and update safety data sheets as results come in, always looking to balance the compound’s value with the risks.
Trends in synthetic methodology push for more sustainable, less hazardous routes to compounds like 1-Methyl-4-Chloropiperidine. Interest stays strong where new piperidine derivatives drive advances in CNS drug discovery or agrochemical innovation. Chemists imagine greener synthesis, improved selectivity, or automated reaction monitoring. Startups search for functionalized piperidines that could stand in, inspired by its success in pharmaceutical and crop science labs. As regulations get stricter on old-school halogenated compounds, pressure mounts for safer substitutes or improved containment. For the foreseeable future, its unique structural features guarantee it a place in the toolbox of those chasing innovation in fine chemicals.
Not everyone wakes up thinking about piperidines, but once you’ve skinned your knuckles in a chemistry lab, the name brings up all kinds of images. 1-Methyl-4-chloropiperidine catches your attention because names carry clues. The piperidine ring, a six-membered ring with five carbons and a nitrogen, shows up in a ton of drug molecules and fine chemicals. Add a methyl group at the nitrogen (the “1” position), hang a chlorine off the fourth carbon, and you get a clear picture of a molecule with a little attitude.
Drawing it on a notepad brings back memories of organic chemistry exams. Imagine a six-sided ring—nitrogen at one corner, carbons at the rest. On the nitrogen, stick a methyl group (–CH3). Walk three carbons around the ring to carbon number four, and there’s your chlorine atom. The chemical formula tells a quick story: C6H12ClN. It’s simple enough at first glance, but there’s a lot tucked behind those letters.
Organic chemists talk a lot about “functional groups” for good reason. Swap a hydrogen out for a chlorine, push a methyl group onto a nitrogen—these tweaks change the way a molecule behaves. A methyl group on the nitrogen makes the compound less basic and more lipophilic, which means it will handle fat and water differently. Chlorine on the ring can slow down how the body breaks it down, or shift the way it interacts with other reactants.
Drug research relies on these little substitutions. Piperidine is part of several medications. Change the ring just a bit and you might get a new sedative, a painkiller, or a precursor to an entirely new class of chemicals. In the history of pharmaceuticals, many breakthroughs sprang from swapping a single atom for another.
I’ve seen smaller labs running reactions with simple piperidine derivatives because they’re handy building blocks. Chemists use 1-methyl-4-chloropiperidine as a starting point to build more complex molecules. These kinds of intermediates don’t always get headlines, but they enable the creation of dyes, pesticides, and medicines. If you look at a pharmaceutical synthesis pathway, you’ll see chemicals like this one bridging the gap between raw material and finished drug.
Lab safety always comes up when handling any compound with chlorine on a ring. Chlorinated organics sometimes show extra toxicity or environmental risk. Gloves and fume hoods aren’t optional. I remember a discussion with an environmental chemist who explained the hassle of disposing of halogenated waste. That’s a real-life cost that gets wrapped into the overall risk assessment for using these raw materials.
Students often ask if a structure like this can become something bigger. The answer points toward innovation. Chemists can use 1-methyl-4-chloropiperidine to craft molecules not just for medicine, but for materials science or agrochemicals. To keep making progress, research needs to balance utility and safety—making sure new derivatives don’t bring more harm than good.
Seeing how a simple change to piperidine’s ring creates a ripple effect through science and industry, you get a sense that every atom has purpose. It’s not about memorizing formulas, but about realizing each piece plays a role in the big, creative puzzle of chemistry.
I remember glancing at a bottle labeled “1-Methyl-4-Chloropiperidine” on a shelf years ago, surrounded by papers full of hand-scribbled notes. At first glance, it just looked like another obscure compound, one only folks in the industry might care about. The reality turns out to be a lot broader. This molecule, with a mouthful of a name, pulls a fair bit of weight behind the scenes in chemical manufacturing and research. Not many people outside the field talk much about the different piperidine derivatives, but 1-Methyl-4-Chloropiperidine has earned its reputation for being useful in places we’d never expect.
I’ve seen firsthand just how crowded the search for new drugs can get. Chemists break and build molecules like puzzle pieces, searching for that one combination that could treat a stubborn illness. Here is where 1-Methyl-4-Chloropiperidine comes into play. It works as a handy starting material or intermediate — the skeleton that chemists use to create new drugs, especially those dealing with neurological conditions. For example, piperidine rings pop up in many medications, including antipsychotics and painkillers. Adding a methyl or chloro group, like this molecule has, lets researchers fine-tune how these drugs behave inside the body. Small tweaks at the atomic level completely change a drug’s effectiveness or how long it lasts in the body.
I once talked to a colleague deep in the world of agrochemicals, trying to make crops hardier against bugs and disease. The same knack for forming strong chemical bonds gives 1-Methyl-4-Chloropiperidine a reliable role here, too. You see, chemists reach for this compound during the early steps of making certain pesticides and herbicides. These finished products need to work predictably, breaking down at just the right speed in the environment. Small changes in the basic ingredient alter toxicity or persistence—sometimes making the difference between clearing a pest and harming the harvest.
I’ve heard folks in the specialty chemicals business talk about “cost per kilogram” as often as athletes discuss training splits. Production runs on efficiency, so it makes sense that 1-Methyl-4-Chloropiperidine attracts attention as a practical choice for making dyes and new synthetic materials. Its chemical behaviors allow for quick reactions, which means more product and less wasted time. This matters in factories where downtime eats into profit, and delays can stall projects that depend on tight schedules.
Anything with potential to do good will also need folks keeping an eye on safe handling practices. 1-Methyl-4-Chloropiperidine isn’t sold over the counter. Anyone working with it wears gloves, goggles, and follows sharp protocols, since piperidine compounds in general carry health risks. Regulators and manufacturers alike keep tabs on supply chains to prevent leaks into places where the chemical doesn’t belong. Past incidents involving piperidine derivatives in illicit labs have shaped tracking and paperwork rules. Responsible sourcing, solid storage, and sound disposal methods will always keep this compound out of the wrong hands.
Every time I look at that old lab bottle now, it’s a reminder that chemistry’s building blocks rarely stand alone. Behind each new pain reliever, cleaner field, or even colorfast fabric, there’s usually a story packed with trial, error, and clever ways to repurpose common materials. Innovations depend on more than just chemistry—they rely on up-to-date education, regulation that keeps risk in check, and a steady line of communication between those in the lab and those on the shop floor.
1-Methyl-4-Chloropiperidine might sound like just another lab chemical, but its hazards don’t blend into the background. It’s flammable, produces fumes, and calls for real respect when it gets pulled out in the lab. I’ve worked in settings where one slip-up with a similar compound left folks scrambling and the safety officer frowning for weeks. This one doesn’t hand out second chances either.
I’ve learned that keeping this compound left on a regular lab shelf spells trouble. Its volatility means it drifts into the air, especially if the container leaks. The first step: screw lids on tight every time. I never trust a crusty old cap that looks half-melted. Clear labeling keeps confusion out—someone confusing this bottle with something less risky? The thought makes me break out in goosebumps.
This chemical prefers a cool, dry cabinet, away from direct sunlight or sources of heat. I’ve seen a bottle stored too close to a window heat up and start sweating, with pressure building inside. Not only is that stressful, but sometimes these vapors lead to explosions. Flammable storage cabinets earn their keep in labs just for moments like this. I also steer clear of acids and oxidizing agents when finding a spot for 1-Methyl-4-Chloropiperidine. Mixing those doesn’t end well.
A locked storage area adds another layer of protection. Sometimes cycles change, new faces show up, and not everyone knows what’s tucked away. I’ve seen janitors come close to opening containers out of curiosity, missing the warning label. Keeping a lock on that door means a lot less to worry about.
Putting on gloves and goggles isn’t some empty ritual with this compound. One careless touch leaves skin burning. One whiff without a mask and you’re coughing for hours. Good ventilation is personal insurance. I always keep work with volatile chemicals inside a fume hood. I’ve opened flasks outside one before and found out quickly what smart lab design is all about.
Spills don’t wait for a convenient time. I keep absorbent materials and neutralizers within reach. My old mentor always stressed the panic factor: “If you don’t plan for cleanup, cleanup plans itself—with chaos.” That holds true every morning I walk into the lab. After work, I wash up—always, no excuses.
Small-volume use beats pouring from big bottles. Splitting stock into smaller containers lowers the impact if something gets knocked over. Keeping chemical logs gives everyone a fighting chance if something happens. Once, a broken record left folks clueless about where a lost bottle could be—nobody wants to repeat that.
Disposal is no cut-corners job. This isn’t trash for the regular bin. I always check local regulations or hand it off to trained waste vendors. Costly? Maybe. Worth the price of safety? Every time.
Gaps in storage or handling pop up most often during busy stretches or after staff changes when training gets rushed. Refresher courses every quarter help anchor procedures into everyday practice. Quality doesn’t come from luck. It grows from habits, from people watching each other’s backs, and from being stubborn about checking every detail.
Chemicals like 1-Methyl-4-Chloropiperidine don’t always get the attention they probably deserve, especially outside the lab. Purity plays a massive role in their reliability. Most suppliers stick to 97% or 98% purity for this compound. I’ve handled a lot of bottles that claim high numbers—sometimes you see “analytical grade” slapped onto the label, setting expectations. But those two numbers, 97% or 98%, often become a sort of minimum standard for anyone hoping their reactions don’t run off the rails. Pharmacy developers, chemical engineers, and even university students all seem to agree that you can’t trust your results on some mystery mix loaded with byproducts. Higher purity brings more predictable reactions, fewer headaches, and less waste at the end.
The difference between 97% and that rare 99%+ batch might seem small on paper, but it adds up quickly in a real flask. Labs that work with sensitive syntheses don’t gamble—they’ll pay extra for the top purity. Lower grades mostly slip into pilot projects or cases where a process can handle a tiny side impurity. Major commercial suppliers, including Sigma-Aldrich and Alfa Aesar, list 1-Methyl-4-Chloropiperidine around that same 97%–98% range, so you know those numbers match industry reality.
Let’s talk packaging. Nobody wants a kilogram of a pungent piperidine derivative sitting on their shelf if they’re running small-scale work. Most labs stick with small packs, usually 1 gram or 5 grams. These usually arrive sealed in glass bottles—plastic could cause trouble if you need to store it for a while, and glass doesn’t tend to leach. It’s easy to keep these bottles tucked inside a secondary container or glove box. Some companies offer 25-gram bottles for bigger jobs in research or short-run production. Working in a place that switches up their protocols every few months often means the medium size—10 or 25 grams—makes sense; it’s not too little, not a wasteful bulk order.
Bulk orders are a different story. Manufacturing outfits or those working with kilo labs can get half-kilo and one-kilo bottles, occasionally drums. From what I’ve seen, few order a kilogram unless their operations already demand strict record-keeping and heavy turnover—tracking and handling become issues for anything sitting open for more than a few days. And once you get into these bigger quantities, suppliers usually insist on proper paperwork, special hazmat shipping, and even certificates of analysis for each shipment.
Anyone using 1-Methyl-4-Chloropiperidine quickly learns storage and handling are almost as important as the grade itself. Leaky caps, light exposure, or even a poorly ventilated shelf can turn an expensive chemical into shelf junk way before the expiry date. In my experience, most labs could do better labeling and organizing their supply. Barcode inventory systems and decent chemical management software do wonders in avoiding wasted stocks, surprise shortages, or dangerous mix-ups with similar-looking bottles.
Suppliers could help out by offering more clear, single-use packaging for ultra-small purchases, especially as interest in high-precision synthesis grows in startups. They might also pilot tamper-evident tabs so buyers know a bottle hasn’t been cracked open at some point on its journey. Small steps like this would lower risk, cut waste, and avoid those rough mornings where you realize the bottle you need is either empty or ruined. If suppliers keep purity at 98% or higher, and offer 1g, 5g, 25g, and 100g sizes, most research and commercial outfits will keep coming back—and keep turning out solid, reproducible work.
Anyone working with chemicals like 1-Methyl-4-Chloropiperidine knows the search for a reliable supplier never gets easier. Markets shift, regulations change, and the phone calls just keep rolling. Last month, I was tracing down a rare intermediate for a client, and landed on this very question: “Can you get 1-Methyl-4-Chloropiperidine shipped fast, and do you have the paperwork?” Behind that simple ask, there’s a mash-up of industry realities most headlines rarely catch.
Supply depends heavily on compliance and red tape. A lot of chemical suppliers hold inventory close, especially for compounds used in pharmaceutical or agricultural research. 1-Methyl-4-Chloropiperidine isn’t the sort of solvent that sits on everyone’s back shelf. Production capacity skews toward larger buyers, and unless you’re placing consistent, sizeable orders, getting your shipment “promptly” can turn into a waiting game. Not to mention, international buyers often stumble into import controls or unexpected paperwork bottlenecks.
Research timelines—especially in pharma and advanced materials—don’t wait around for logistics. A missed deadline on chemical delivery can stall an entire project. I remember in 2021, my contacts at a biotech firm nearly missed a key project milestone over a delayed shipment from Asia. One supplier’s idea of “prompt” meant three weeks, which simply doesn’t fly for people running on tight grants. Time truly is money in these labs.
Every real buyer asks about the certificate of analysis (CoA). I’ve lost count of the number of times I’ve been pitched a reagent with little to show beyond a generic product sheet. That’s a red flag. A proper CoA breaks down the batch’s purity level, outlines impurities, and can even show residue solvent content. I always insist suppliers provide recent, lot-specific CoAs—not vague templates—since the lab teams I work with refuse to put unknowns in their scale-up equipment. Without that document, you’re gambling with your results and potentially risking big compliance headaches down the line.
From my experience, the best approach is building close partnerships with established suppliers—never settle for transactional relationships. Suppliers with years in the field, or proven documentation trails, usually deliver on time and respond quickly about real-time inventory. If you’re sourcing 1-Methyl-4-Chloropiperidine, try reaching out to two or three sources at once, making it clear you need a CoA before PO approval. I’ve seen labs form pretty informal “supplier watchlists,” noting who comes through and who falls short on both timing and paperwork. Sharing information inside the community makes all the difference.
Transparency remains the missing piece. Online inventories are often outdated, shipping timelines rarely match website claims, and some suppliers hesitate to provide full documentation until late in the process. If suppliers can standardize real-time stock updates, and automate the sending of batch-specific CoAs, buyers would spend less time chasing e-mails and more time in the lab. I’ve heard talk of peer-reviewed supplier ratings, which might nudge the market toward more reliable delivery and documentation. Until then, phone calls, email follow-ups, and peer recommendations still rule the day.
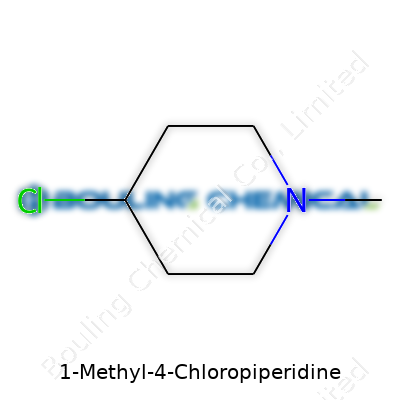
| Names | |
| Preferred IUPAC name | 4-Chloro-1-methylpiperidine |
| Other names |
N-Methyl-4-chloropiperidine 1-Methyl-4-chloro-piperidine 4-Chloro-1-methylpiperidine |
| Pronunciation | /ˈwʌn ˈmɛθɪl fɔːr ˈklɔːrəʊ paɪˈpɛrɪdiːn/ |
| Identifiers | |
| CAS Number | 930-28-9 |
| Beilstein Reference | 1718731 |
| ChEBI | CHEBI:19206 |
| ChEMBL | CHEMBL152736 |
| ChemSpider | 113889 |
| DrugBank | DB08399 |
| ECHA InfoCard | 22-212-498-6 |
| EC Number | 628-858-8 |
| Gmelin Reference | 81868 |
| KEGG | C18603 |
| MeSH | D058345 |
| PubChem CID | 12509569 |
| RTECS number | TM3150000 |
| UNII | 07V1Y66I8G |
| UN number | UN3431 |
| Properties | |
| Chemical formula | C6H12ClN |
| Molar mass | 133.62 g/mol |
| Appearance | Colorless to yellow liquid |
| Odor | fishy |
| Density | 0.967 g/mL at 25 °C (lit.) |
| Solubility in water | Slightly soluble |
| log P | 1.79 |
| Vapor pressure | 0.6 mmHg (at 25 °C) |
| Acidity (pKa) | pKa = 10.56 |
| Basicity (pKb) | 3.79 |
| Magnetic susceptibility (χ) | -71.1·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.484 |
| Viscosity | 1.4 mPa·s (20°C) |
| Dipole moment | 2.49 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 352.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -99.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3932.3 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H314 |
| Precautionary statements | P210, P261, P280, P301+P312, P305+P351+P338, P337+P313, P403+P233 |
| NFPA 704 (fire diamond) | 2-3-0 |
| Flash point | 46°C (closed cup) |
| Autoignition temperature | 215 °C |
| Explosive limits | Explosive limits: 1.4–11% |
| Lethal dose or concentration | LD50 (oral, rat): 175 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat 178 mg/kg |
| NIOSH | ST0771390 |
| PEL (Permissible) | Not established |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
4-Chloropiperidine 1-Methylpiperidine 1-Methyl-4-piperidone 4-Chloro-N-methylpiperidine Piperidine |