My journey with 1-(M-Chlorophenyl)Piperazine, often called mCPP, starts in the 1970s, a time when the demand for new compounds in both research and industry grew fast. Originally, chemists explored this molecule as part of the push to better understand piperazine derivatives, which seemed to offer a unique window into neurochemistry and drug development. Over the years, mCPP found a place not only in labs but in the hands of regulatory bodies watching for recreational misuse. That historical thread — research curiosity tangled with safety concerns — shapes nearly every discussion about mCPP today.
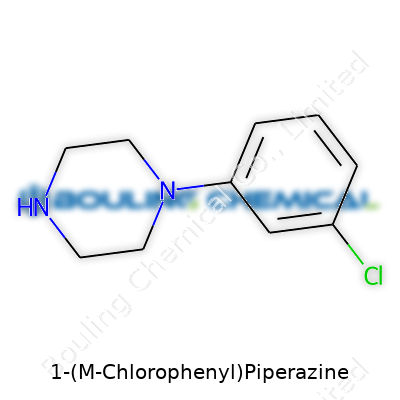
Scientists classify mCPP as a psychoactive piperazine derivative. Structurally, it brings together a piperazine ring with a chlorinated phenyl group, creating a molecule that's found its way into pharmaceutical research and, less desirably, into illegal drug formulations. It isn’t something one stumbles upon in a pharmacy; its presence is typically linked to intermediate use in organic syntheses or as a standard in laboratory settings. While once approached as a potential antidepressant candidate, today it functions mainly as a research chemical.
As white crystalline solid at room temperature, mCPP melts around 248 degrees Celsius. It dissolves slightly in water, far better in organic solvents like methanol or ethanol, which lines up with its moderately polar structure. The molecular weight lands just over 196 g/mol. Chemical reactivity follows typical path for piperazine bases: it forms salts easily and undergoes standard aromatic substitutions on the phenyl ring. Density, vapor pressure, and similar numbers often land in standard technical documentation rather than in experience, but handling mCPP always means dealing with a fine particulate — easy to lose, hard to clean up without good ventilation and protective gear.
Labels on laboratory bottles or suppliers’ catalogs for mCPP always spell out the CAS number, formula (C10H13ClN2), and purity. In professional circles, researchers expect nothing less than 98% purity, most often as a hydrochloride salt for stability. Safety labels warn of skin and eye irritation risks. They also note neuroactive effects, which means even unintentional contact in small amounts could lead to acute symptoms. I have seen labs flagged for not following hazard communication standards, so clear, prominent labeling never feels like overkill.
Any chemist prepping mCPP tends to rely on nucleophilic aromatic substitution. They’ll treat m-chloronitrobenzene with piperazine under basic conditions, reduce the nitro group, and purify the resulting mCPP by acid-base extraction. Each step demands precision; impurities linger if reactions drift from narrow temperature or pH windows. It doesn’t scale up easily without automated controls, and any slip-up risks not just lost batches but regulatory headaches due to precursor status. I recall projects where a single unscheduled inspection by environmental safety teams delayed work for weeks.
Chemists often use mCPP as a springboard for more complex compounds. Attaching various groups to the phenyl ring, or swapping the piperazine for other cyclic amines, leads to a large family of related bioactive molecules. Catalytic hydrogenation, halogen exchange, and even simple alkylation all see action in research. These transformations help map out serotonin receptors or test new psychiatric drugs in vitro. Testing modifications always comes with regulatory oversight, since every new analog could trigger legislative reviews or scheduling proposals in many countries.
Over the years, mCPP collected a heap of names: 1-(3-Chlorophenyl)piperazine, pCPP, 1-(meta-chlorophenyl)piperazine, 3-chlorophenylpiperazine. They all point to the same core compound but pop up in catalogs, research papers, and agency alerts alike. Keeping track feels like chasing a hydra — each new region or supplier spawns a slightly tweaked name, sometimes just to skirt scheduling laws. This wide spread of synonyms often confuses new researchers or confounds customs officials, especially when import or export regulations come into play.
Lab experience with mCPP taught me to take exposure risk seriously. No exceptions for short jobs or quick transfers — gloves, goggles, and fume hoods are non-negotiable. Spill cleanup plans require dedicated containers and solvents. Handling powder form mandates respiratory protection. Both short-term symptoms like nausea and headaches, and long-term neurotoxicity concerns, drive tight procedural controls. Disposal guidelines demand incineration or chemical deactivation per local environmental standards. Labs that cut corners not only endanger staff but risk losing research grants and licensing.
Pharmaceutical research dominates mCPP’s legitimate world. Its role as a serotonergic agent made it a vital probe in mapping mental health disorders from depression to anxiety. Some antipsychotic and antidepressant drugs use it as a synthetic precursor or metabolic reference compound. Beyond pharma, forensic labs track mCPP to flag illegal designer drugs. Its structural flexibility also invites use as a stepping-stone in creating novel chemical libraries, necessary for high-throughput drug screening. Regulation keeps shrinking its open applications but doesn’t erase its usefulness where strict oversight exists.
In my own projects, mCPP often represented both a tool and a hurdle. On one hand, studying how it binds to serotonin receptors helped clarify how similar psychoactive drugs work in the brain. On the other, increased regulatory attention turned mCPP-centered projects into compliance headaches. Research trends show a clear shift toward controlled environments: animal studies, metabolic fate analysis, and environmental impact studies, all carried out under watchful review boards. Advances in analytical chemistry, like mass spectrometry, let scientists track trace metabolites, offering new insights into both drug action and environmental pollution.
Toxicity stands as one of the main barriers mCPP faces as anything more than a reference chemical today. Reports tie the compound to side effects ranging from agitation and headaches to severe neurological symptoms. Accidental overdoses, particularly among recreational users, highlighted its unpredictable potency and risk profile. Academic studies dig into its mechanism: serotonin syndrome and cardiovascular strain lead the list of dangers. Agencies use animal and cellular models to map where these risks emerge and how long-term exposure might impact neural function. Real-world data and clinical case reports add practical weight to lab findings, keeping the focus on limiting exposure and tightening sale restrictions.
Looking ahead, mCPP walks a narrow line. In drug research, its legacy lies in helping map vital neurotransmitter pathways, but the shadow of risk never fades. Stricter laws in different nations close doors for open research, but targeted medical work — central nervous system disorder studies, for example — will likely keep some demand alive. Analytical chemists and forensic scientists continue developing better methods to detect it, searching for both environmental traces and markers of illegal use. Continued R&D could uncover metabolites or safer analogs with less toxicity, but every proposal faces tough ethical and regulatory screening. My experience confirms that any future for mCPP depends on transparent risk communication, ongoing safety audits, and a willingness to pivot as science and regulation both move forward.
1-(m-Chlorophenyl)piperazine, often called mCPP in research circles, pops up in more conversations than folks might expect. At first glance, there's nothing especially interesting about this white powder. Dig a bit deeper, and things get more complicated. Seems simple, but once it starts getting mixed into pills or used in labs, those chemical chains get tangled up with real-world problems.
Anyone who’s done time around pharmaceuticals or heard stories from friends in health care has probably come across mCPP, sometimes for the wrong reasons. It’s not a medication that gets handed out for a sore throat or high blood pressure. Researchers study it mostly as a tool, sort of like a probe, to help understand how the brain works—especially the parts involving serotonin. Serotonin’s one of those neurotransmitters that affects how people sleep, eat, and feel, so figuring it out gets a lot of attention. Scientists use mCPP to mess with serotonin systems in lab animals or in carefully controlled studies on humans, hoping to shed light on depression, anxiety, and migraines.
I’ve seen more than a few reports about people ending up in the emergency room, thinking they’ve taken one thing and winding up with something entirely different in their system. mCPP isn’t some party drug made for good times, but it shows up as a contaminant in the illegal drug scene, especially in fake versions of MDMA (ecstasy). Dealers cut corners and mix in whatever bulk chemicals they can get, and mCPP slides in because it’s cheaper and available. Effects range from headaches and nausea to unpredictable panic and agitation. Not exactly what festival-goers have in mind.
Here’s something I learned reading European Monitoring Centre bulletins and old toxicology notes—mCPP traces turn up in pills seized at borders or club raids all the time. Unlike MDMA, it tends to leave people with headaches and a racing pulse, far from a euphoria. This matters for public health, not just because of what’s in the drugs, but because mCPP complicates efforts to predict or treat reactions. If you’re lying in a hospital bed and the medics guess wrong about what you took, treatment gets dicey fast.
Trying to cut down on unwanted mCPP in recreational drugs means stricter controls on chemical precursors, sharper customs checks, and more accurate field-test kits at music venues. Making sure hospital staff and first responders can rapidly identify what’s in a patient’s system changes outcomes. Education for anyone at risk helps too. Harm reduction teams have pushed for better pill testing and honest conversations, not moral panic.
Though mCPP earns a bad reputation on the street, its story started at the lab bench. Chemical companies sell it for legitimate research uses. Medical science, especially in psychiatric drug development, needs small-scale trials using chemicals like mCPP to figure out the root causes of complex illnesses. Clamping down on sales too tightly might choke innovation in mental health research. Balancing safety and progress needs good policy and real understanding of what’s at stake on both sides of the law.
1-(m-Chlorophenyl)piperazine, often called mCPP, pops up in conversations that usually circle around club drugs, designer chemicals, and tricky shelves of online shops. Some folks know it from the world of pharmaceuticals, as a building block in chemical research or a mood-altering substance that winds up as an ingredient in pills with more familiar names. Still, people ask: can you buy it, or even own it, without ending up on the wrong side of the law?
MCPP lives in a legal gray area. What’s legal in one country looks a world apart from the bans and criminal penalties in another. In the United States, mCPP doesn’t appear specifically on the federal controlled substances list. That doesn’t always mean it’s legal, though. Many states and a few local governments have chosen to ban it. Lawmakers grew tired of synthetic drugs slipping through loopholes, so they passed “analog” laws. If a chemical acts enough like a banned drug, it can be treated as if it is one.
The story looks different in places like the United Kingdom. MCPP is listed as a Class C drug there, lumped together with substances people use and abuse for their effects on the mind. Selling it, buying it, or even holding onto it could mean serious trouble. Several countries in Europe came to similar decisions. They watched designer drugs rise in popularity and came down hard.
Legalities aside, most people rarely stop to think about what’s actually inside the powders and pills they buy online. MCPP often acts as a contaminant in recreational drugs or gets falsely labeled as something else entirely. People taking pills at festivals or clubs sometimes end up with nasty side effects they never saw coming: headaches, anxiety, rapid heartbeat, and worse. When I worked with harm reduction groups, I saw a wave of panic after laboratory testing services started turning up mCPP in places no one expected. Even folks who thought they knew the score found themselves caught off guard.
Medical literature backs up these experiences. MCPP binds to serotonin receptors and can set off unpleasant reactions, even at low doses. In medical research, it’s been used to induce anxiety or other psychiatric responses in volunteers. That’s hardly what anyone is looking for at a party, and it doesn’t get any safer just because you found a way to buy it.
People buy mCPP for different reasons—sometimes out of curiosity, sometimes chasing a high, sometimes trying to keep up with trends shown in online forums. What usually gets missed is that the legal status stays in flux, with enforcement that can be both harsh and unpredictable. Some products hide mCPP behind chemical jargon or flashy branding. Even for those trying to stay informed, it’s easy to fall behind as chemists develop new versions faster than laws get written.
Harm reduction starts with honest conversations about risks, not just legal risks but health and safety, too. Lab testing, clear labeling, and up-to-date information give people better chances to avoid hidden dangers. If there’s demand for mCPP or chemicals like it, ignoring or banning them without education just pushes people into darker corners of the internet. Open discussion and testing services can save lives and help more people stay out of trouble—legal and otherwise.
1-(m-Chlorophenyl)piperazine doesn’t turn up in daily headlines unless someone’s asking tough questions about recreational drugs or chemical research. This compound, known on the street as mCPP, has developed a bit of a reputation. Some people have used it chasing a high, others find it mixed into pills not labeled for it at all. Years ago, I ran into someone who got it in what they thought was something else entirely, and that migraine lasted two days. Knowing what it can do becomes personal fast.
This stuff doesn’t mess around. Folks report headaches—bad ones, the kind that make any plan disappear. Nausea and vomiting tap in quickly. That’s not too rare, either. mCPP seems to tweak neurotransmitter levels, especially serotonin, leading to symptoms almost like mild serotonin syndrome: flushed skin, sweats, agitation, even sweating through your shirt in a cool room. According to research published in Drug and Alcohol Dependence, a lot of people end up sick to their stomach after exposure, and it isn’t just in party scenarios.
I’ve heard from ER nurses that tachycardia and high blood pressure show up, too. It’s not surprising, given what we know about its action on the body. Sometimes pupils dilate, hands get clammy, and dehydration sneaks up, especially if someone keeps moving or doesn’t eat. Unlike an average recreational drug, the risk of seriously unpleasant side effects seems higher.
On the mental side, anxiety takes center stage. Some people get panic attacks—real, full-on panic where logic feels far away. Mood swings aren’t far behind. In my old social worker days, a young woman told me she felt like the world would never go back to normal after taking what was later found to be mCPP. Her sleep splintered; she couldn’t string together four hours of rest for a week.
Hallucinations and paranoia pop up in some cases, hitting those with a history of mental health struggles the hardest. Most people don’t expect a substance that’s chemically related to some antidepressants (like trazodone or nefazodone) to spark psychosis, but it’s happened in case reports. There’s no escaping the fact that for some, a bad night can turn into a bad month.
Those with mood disorders or a family history of mental illness run a heavier risk. Children and teens end up in emergency rooms if they get exposed accidentally—an issue that’s grown as some designer drugs copy cat mCPP’s structure. Undiagnosed cardiac issues mean danger when blood pressure and heart rhythms stray from normal. For every person who shrugs off mild symptoms, another lands in trouble quickly.
Education matters. If health class skips over what random chemicals in party drugs can mean, that gap shows up in emergency calls. Community drug checking services are a start—these groups test street pills so people know what they’re holding. Doctors can ask about mCPP as a possible culprit when they see someone with strange side effects, especially if prescription meds don’t explain everything.
Pharmacies and researchers need to watch shipments. Regulation across countries isn’t uniform, so something banned in one place might drift into another. Friends watching out for each other at parties or clubs make a real difference, too. That’s what stops a “bad batch” from becoming tomorrow’s headline.
Anyone with experience in a lab or industrial setting recognizes caution comes first with chemicals like 1-(m-Chlorophenyl)piperazine. This compound has found its way into research, pharmaceuticals, and sometimes, the less savory world of recreational misuse. Its structure puts it firmly in the class of chemicals that can bring real risks without proper handling. In such situations, respect for the hazards isn’t optional; it’s just part of the job.
People get into trouble when harsh chemicals are stored wherever there’s space or when opened containers get shoved under a bench. Over the years, I’ve seen labs get shut down by leaks from mismanaged containers. Regulations exist for a reason. Shortcuts usually bring more trouble than convenience, especially with chemicals that can irritate, burn, or release toxic fumes like this one.
Some workers feel tempted to transfer small quantities into food containers or leave labels half-peeled and unreadable. Avoiding clear labeling opens the door to accidental exposure. Glass jars that once held peanut butter don’t keep out air or moisture, both of which can react with some organics to release harmful byproducts or turn a powder into a sticky mess. Worse, mixing this compound with acids, oxidizers, or bases can create dangerous reactions—something all chemists learn from early mistakes or close calls.
Store this compound in a place with steady, cool temperatures. Direct sunlight warps containers and speeds decomposition. Humidity creeps in and, in some cases, ruins the chemical or affects the purity of a batch. Tall shelving sounds convenient, but in an emergency, you need to grab chemicals quickly. Keep toxics at eye level or below, and never above your head. My own run-in with a falling bottle reminds me why — broken glass, spilled powders, chaos.
Theft and unauthorized use create big legal headaches. Some people seek out 1-(m-Chlorophenyl)piperazine for misuse. Restricted access isn't about being unfriendly—it protects the public and the team. Store it in a locked cabinet or storeroom. If your workspace uses digital logs or keys for entries, track who accesses what and when. Strange as it sounds, a simple sign-in sheet has, more than once, caught mishandling before it escalated.
Go with containers made for chemical storage—thick glass or sturdy plastics with secure, screw-on lids. The original manufacturer’s packaging gives clues about what works. Make sure each container wears a clear, smudge-free label, including hazard warnings and the date opened. Chemical compatibility charts line the walls of most good labs for a reason. Spraying a label with water-proof coating helps, especially in humid areas.
Even with every precaution, spills happen. Kits should sit near storage—absorbent pads, gloves, eye protection, and disposal bags. Everyone working with the compound needs quick refresher training, so panic doesn’t take over if something spills or splashes. Emergency contact numbers and procedures ought to hang where anyone can see them without hunting. Better once too often than a minute too late.
Disposal is part of storage. Don’t let unwanted chemicals build up; periodic checks lower risks. If you spot signs of leaks, deterioration, or crystal buildup, handle the problem swiftly. Local agencies offer supports, from hazardous waste pickup to training. Old chemicals aren’t just clutter—they can turn nasty as they age.
It isn’t about fear—it’s about respect. Reliable storage habits for chemicals like 1-(m-Chlorophenyl)piperazine protect people, property, and keep businesses or laboratories running without interruption. These small choices build a safer work environment, one decision at a time.
1-(m-Chlorophenyl)piperazine, or mCPP as chemists call it, has surfaced in both clinical research and, less safely, on the street. Some folks hear about it as an impurity in recreational drugs, others recognize its role as a pharmaceutical research tool. mCPP acts as a psychoactive agent and interacts with several serotonin receptors in the brain. This profile puts it in a gray zone—a lab chemical, but not a medication approved for health purposes.
Doctors have not prescribed mCPP for any health condition. Drug agencies, including the FDA and EMA, have not given it the green light as a safe or effective treatment. Put simply, there’s no label, no packaging, no doctor’s signature—just scattered reports and lab notes describing its effects.
No standard dosage exists for this chemical—no matter the method or reason. Any numbers floating around online come from risky self-experimentation or old research, not from controlled or peer-reviewed clinical trials. People snooping for “proper doses” hit a dead end. In a hospital or research setting, mCPP shows up as a tool to mimic migraine symptoms for testing headache drugs. Even there, scientists keep extreme care while handling it, measuring out amounts precisely and under heavy oversight.
Some reports talk about taking mCPP orally, others mention capsule form for research trials. Recreational exposure often involves swallowing powder or ingesting a tablet. An important point: doses that cause mind-altering effects closely clash with those that harm the body. Too much mCPP doesn’t just mean a bigger high—it can bring serious problems. Agitation, panic, shivering, sweating, heart racing, and even seizures or psychosis have all shown up among those who took a gamble with this compound.
Drug markets often mix mCPP with other substances, especially ecstasy pills. Users can’t identify strength or purity without advanced testing equipment. Pills bought off the street might carry variable amounts. Sometimes, a single dose causes stronger side effects than expected, leading to emergencies.
In research, subjects reporting negative results make it clear—mCPP comes with strong risks. Headaches, anxiety, and mood disturbances crop up more often than pleasant effects. Studies tie mCPP to triggering migraine attacks and panic symptoms in susceptible people. Brain and heart health both suffer under its influence.
People deserve factual information, not half-truths from forums or word-of-mouth guesses. Harm reduction groups play a critical role, offering drug-checking services and education around hidden ingredients. Physicians, toxicologists, and drug counselors encourage steering clear of experimental chemicals lacking medical backing. Some push for more widespread and accessible drug testing at events or clinics, helping folks steer away from unknown risks. Open conversations about mental health, substance use, and healthy coping strategies matter far more than chasing after an unregulated, unpredictable chemical.
| Names | |
| Preferred IUPAC name | 1-(3-chlorophenyl)piperazine |
| Other names |
mCPP meta-Chlorophenylpiperazine 1-(3-Chlorophenyl)piperazine |
| Pronunciation | /ˈwʌn ɛm ˈklɔːrəʊˈfiːnɪl paɪpəˈreɪziːn/ |
| Identifiers | |
| CAS Number | 34803-66-2 |
| Beilstein Reference | 120927 |
| ChEBI | CHEBI:74755 |
| ChEMBL | CHEMBL1427 |
| ChemSpider | 13319 |
| DrugBank | DB00720 |
| ECHA InfoCard | ECHA InfoCard: 100.121.371 |
| EC Number | 202-853-6 |
| Gmelin Reference | 107171 |
| KEGG | C13947 |
| MeSH | D03.633.100.221.173 |
| PubChem CID | 71626 |
| RTECS number | TM3675000 |
| UNII | X1ZZY9QK09 |
| UN number | UN3439 |
| CompTox Dashboard (EPA) | DTXSID2020089 |
| Properties | |
| Chemical formula | C10H13ClN2 |
| Molar mass | 212.68 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.13 g/cm³ |
| Solubility in water | Slightly soluble in water |
| log P | 2.48 |
| Vapor pressure | 5.2E-3 mmHg at 25°C |
| Acidity (pKa) | pKa = 9.73 |
| Basicity (pKb) | 5.52 |
| Magnetic susceptibility (χ) | -74.7×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.584 |
| Viscosity | Viscous liquid |
| Dipole moment | 3.73 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 220.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | ΔfH⦵298 = 74.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3897.7 kJ/mol |
| Pharmacology | |
| ATC code | N06AX19 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS06, GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P301+P312, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-3-0 |
| Flash point | 99°C |
| Autoignition temperature | > 530°C |
| Lethal dose or concentration | LD50 (oral, rat): 210 mg/kg |
| LD50 (median dose) | LD50 (median dose): 210 mg/kg (mouse, oral) |
| NIOSH | JN9275000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 ppm |
| Related compounds | |
| Related compounds |
1-Phenylpiperazine para-Chlorophenylpiperazine 2-Chlorophenylpiperazine 3-Trifluoromethylphenylpiperazine 2-Methylphenylpiperazine |