Chemists have poked and prodded the imidazole ring for over a century, but adding an isopropyl group didn’t catch major attention until progress in organic synthesis made it easier and cheaper. Researchers in the mid-20th century found the imidazole platform chemically versatile, showing up in nature—think histidine or vitamin B12. R&D ramped up as lab processes got sharper and a need for specialty building blocks grew in pharmaceuticals and material science. As scientists tinkered, 1-isopropylimidazole emerged for applications needing both an accessible N-heterocycle and a tweakable side chain. This chemical offers more than just theory; it adds new possibilities for customizing molecules in a lab, expanding what chemists and manufacturers can accomplish.
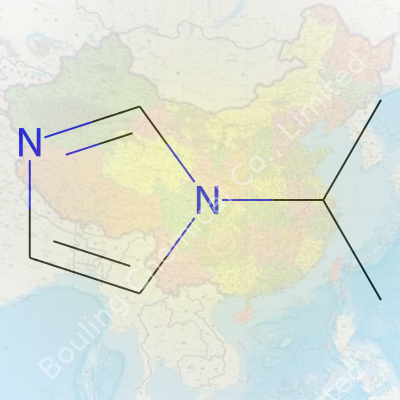
1-Isopropylimidazole presents as an organic compound with a backbone similar to standard imidazole, but it’s the isopropyl group at the 1-position that gives it unique reactivity. This ring-and-chain combo supports all sorts of operations in synthesis routes. In practice, I’ve found it becomes a go-to intermediate when classic imidazole sometimes falls short. As a white to off-white crystalline solid, it slips easily into different mixtures or processes, pairing up with everything from salt-forming acids to halogens, and allowing for a broad range of derivative production.
1-Isopropylimidazole stands out for its manageable melting point, usually hovering around 60-65°C, and a boiling point that stays comfortable enough to handle in standard glassware—roughly 235-240°C at atmospheric pressure. At room temperature, it has moderate solubility in common organic solvents like ethanol, acetone, or toluene, but it also tolerates water, which can sometimes make purification trickier but opens up greener synthetic options. This compound features an imidazole ring system known for nitrogen-based hydrogen bonding, while its isopropyl group adds a bit of hydrophobic character, influencing reactivity and partitioning when you mix it with other chemicals. Its molecular formula, C6H10N2, keeps it compact, and its stability resists decomposition in standard storage conditions.
On supplier data sheets and labels, you’ll spot 1-isopropylimidazole usually listed as “≥98.0% purity” or even higher, depending on regulatory or research requirements. Most bottles carry a CAS Number (of 32298-41-8), and labeling often details physical state, hazard warnings, and recommended storage conditions—typically in a tightly closed container, away from sources of ignition or strong oxidizers. I’ve seen material safety data sheets flag standard precautions common for low-volatility organics: gloves, goggles, a fume hood—basic but not negotiable. Some technical sheets add GC-MS data or NMR spectra to confirm lot quality. Reliable labeling can’t be overlooked; getting a misidentified intermediate means lost time, wasted budget, or ruined experiments.
Lab synthesis of 1-isopropylimidazole commonly starts with imidazole itself, taking advantage of its active nitrogen sites. The classic route I’ve used begins with alkylation—reacting imidazole potassium salt with isopropyl bromide or isopropyl chloride—under controlled temperature, often in a polar aprotic solvent like DMF. Reaction yields benefit from careful TLC monitoring and subsequent extraction, followed by vacuum distillation or recrystallization from a suitable solvent. This method’s cost and convenience make bulk production feasible, especially where large-scale pharmaceutical or materials development plays out.
The imidazole ring system in 1-isopropylimidazole opens doors for a slate of reactions. The isopropyl group at the N1 position tends to block those sites for further N-alkylation but leaves the rest of the ring accessible for substitution, oxidation, or metal-catalyzed coupling. My lab work often sees this compound participating in acylation or sulfonation reactions, which can tune solubility or introduce chromophores. In some polymer chemistry applications, it provides a stable pendant group for thermal or electrical performance boosts, as imidazoles tend to form strong complexes with metals or other heterocycles.
Look around catalogs or research papers and you’ll see this compound tagged under a few aliases. Besides 1-isopropylimidazole, alternative names like “N-isopropylimidazole,” “2-(1-Methylethyl)imidazole,” or even abbreviations like iPrIM come up. These synonyms aren’t just semantics; ambiguity in names can lead to confusion in multi-team projects or when ordering for large ongoing studies. Standardization makes a difference, so cross-checking CAS Numbers (32298-41-8) and consulting supplier references always stays on my checklist.
Safety management deserves real attention even for chemicals that haven’t gathered a scary reputation. 1-Isopropylimidazole earns a spot as a low- to moderate-hazard compound, based on available LD50 data and what I’ve experienced with eye or skin irritation upon accidental exposure. Good practice means working in a fume hood, using nitrile gloves and splash protection, and ensuring prompt spill cleanup. Waste gets disposed of as organic solvent or through incineration routes, subject to local regulation. The absence of major combustibility or chronic toxicity lets researchers focus on careful but not nerve-wracking protocols—but that doesn’t mean skipping hazard reviews or OSHA logs.
This chemical doesn’t stay on the sidelines. In drug research, it enters pre-clinical workflows as a building block for antifungal, antiviral, or enzyme-inhibiting candidates. Electrochemistry labs make use of its strong electron-donating imidazole ring while exploring corrosion inhibitors or ionic liquids. The world of resins, polymers, and adhesives also finds value here, particularly for thermal curing agents in epoxy systems, where the side chain optimizes flow and mechanical performance. My own experience with specialty catalysts shows its versatility, producing highly tailored ligands for homogeneous reactions, sometimes outperforming standard imidazole analogs, especially in heat- or moisture-sensitive settings.
New research keeps 1-isopropylimidazole in the spotlight. Recent publications highlight its use in electrochemical sensors, as tweaking the ring's substitution pattern can sharpen sensitivity to molecular analytes. Groups working on green chemistry appreciate its water-solubility potential for environmentally friendly syntheses. I’ve watched colleagues in medicinal chemistry tap into the structure's modularity, quickly generating libraries to screen against disease targets. The growing landscape around molecular electronics pulls in imidazole derivatives, too, given their conductive and chelating potential in nanoscale assemblies.
Toxicological studies for 1-isopropylimidazole are ongoing, though its parent compounds have received much more scrutiny in regulatory evaluations. So far, acute exposures in animal models indicate low risk of systemic toxicity, though repeated skin contact can lead to mild irritation. Researchers tracking environmental fate haven’t flagged it as persistent or bioaccumulative, but comprehensive data covering chronic ingestion or inhalation is still thin. I always suggest erring on the side of caution, using minimum quantities and following established lab hygiene, especially when scaling up reactions or preparing derivatives that could show higher reactivity or unexpected hazards.
Innovation keeps this molecule relevant. As global industries push for better, safer, and more efficient functional chemicals, the story of 1-isopropylimidazole keeps evolving. New surface coatings, next-gen electrolytes, smart polymers, pharma intermediates—all show expanding demand for specialized heterocycles. Upcoming regulatory frameworks around environmental safety and sustainable production could drive greener synthesis routes, possibly embracing biocatalysis or solvent-free chemistry approaches for making this compound. With ongoing R&D into its derivatives and applications, both academic and commercial labs have plenty of ground left to cover, ensuring that 1-isopropylimidazole stays more than just a footnote on the chemical supply shelf.
You probably haven’t heard much about 1-Isopropylimidazole. Still, it keeps factories and laboratories ticking. Out of all the chemicals floating around the industry world, this one doesn’t get much press. Its biggest claim comes from workhorse tasks in organic chemistry and industry. If you open a bottle in a lab, most folks probably wouldn’t bat an eye, but that doesn’t make it any less important. I once saw a batch arrive in a plain plastic drum at a facility—no fancy warnings, no hype. Yet, it was the quiet backbone of a project moving polyurethane research forward.
Chemical reactions often need helpers. 1-Isopropylimidazole acts as a catalyst. That means it speeds up reactions that otherwise grind along slowly. For me, catalysts are kind of like the kitchen gadgets of the chemistry world; you can technically get by without them, but life gets much easier—and tidier—with the right tool.
You’ll see this compound play a big part in making polyurethane foam, special coatings, adhesives, and sometimes pharmaceuticals. Polyurethane foam, the stuff in your memory foam mattress or running shoes, uses this chemical to help the pieces link up and form resilient, springy material. Without the right push, that reaction either never gets going, or takes forever. A reliable catalyst like 1-Isopropylimidazole smooths out the bumps.
Beyond its catalytic knack, 1-Isopropylimidazole works as a solvent or an intermediate for more complex molecules. Labs use it to dissolve things that stubbornly resist mixing. Larger companies use it as a stepping stone; they start with 1-Isopropylimidazole and end up with niche medications or advanced materials a couple of steps later.
Any time someone moves chemicals around in a workplace, risks show up. 1-Isopropylimidazole isn’t the most dangerous stuff in the cabinet, but it’s not a bottle of vitamins either. Contact with skin might cause irritation; spills need a careful clean-up. Regulations around storage and handling can seem fussy, but they matter. I’ve seen corners cut in smaller operations, and trouble never takes long to follow—accidents, lost product, and sometimes injuries.
More importantly, chemical regulations vary wildly from place to place. Where I live, every shipment gets tracked carefully. In some countries, recordkeeping feels more like a suggestion. Gaps like that spell trouble, especially once this compound heads toward more complex synthesis.
Safe handling and environmental care are not mysteries. Folks in the business know how to keep things contained. But budgets squeeze training to the margins, and some companies skip on ventilated storage or proper disposal. Solution? Stronger accountability and regular inspections, mixed with simple, tough training for every person on the ground. Not the sort of tech-heavy fix most consultants hype, but sometimes the basics offer more protection than any fancy device.
As more specialty chemicals reach growing markets, conversations about worker safety and waste stream control need teeth. Making use of 1-Isopropylimidazole can unlock great products, but without old-fashioned vigilance, that progress costs more than it should. So it pays, in my experience, to treat every drum—no matter how quiet or plain—with the full respect it deserves.
1-Isopropylimidazole vendors usually broadcast their numbers front and center, touting purity figures such as 95%, 97%, and, on a good day, 99%. Most buyers—chemists, manufacturing leads, and research students—scan those numbers and move on to price and delivery, but there’s a story beneath the surface that goes beyond single-percentage marketing.
Chemical purity connects directly to the confidence someone has during a reaction or analysis. In the case of 1-Isopropylimidazole, those last few percentage points make a real difference. Impurities bring hidden traits—unwanted byproducts, tough-to-track variables, things that never show up in the protocol but change your outcome or leave doubts in your results. From handling batches in a university lab, I’ve seen how even minor inconsistencies create headaches, with cloudy results and wasted days following up false leads.
There’s no all-knowing judge that stamps bottles with an official “chemically pure” certification. Most of the time, 1-Isopropylimidazole on the market comes labeled with “97%” or “98%” purity, measured using techniques like gas chromatography, HPLC, or NMR. This matters because the ‘other side’ of those percentages—those leftover two or three percent—could include water, unreacted isopropylamine, or other byproducts from the synthesis process. For a researcher running sensitive catalysis experiments or pharmaceutical studies, these small contaminants punch above their weight.
Working with a barely adequate grade, the first thing I notice is unpredictability: color shifts, odd smells, or reactions that seem just slightly off but not so much as to scream “disaster.” Higher grades (usually labeled as “analytical” or “ACS reagent”) cost more, but they buy back your time and trust in the data. Industry buyers watch these numbers not simply for safety but because the small print can mean scaling up a process with or without extra purification steps—each adding cost and risk.
As this chemical moves from beaker to industry, the need for reliable purity stretches beyond scientists and into real-world production. Pharma companies and specialty chemical plants want consistency. Even a tiny trace of a rogue impurity can set off recalls or regulatory headaches. In a place where one impurity can spoil an entire batch or bring operations to a halt, the “purity” figure translates directly to bottom lines and reputations.
I’ve seen occasional blind trust called out when someone bases a big process on a supplier’s certificate of analysis. Sometimes, the actual batch purity falls short of what was billed. Audits or follow-up analyses reveal the real picture—sometimes just water weight, sometimes more troubling unknowns. Reputable suppliers open their data and batch analysis results. Less transparent sellers create more work and worry for everyone down the chain.
For customers who stake projects on dependable ingredients, demanding batch-specific data is essential. Skip the assumption that every lot matches the last. Instead, request gas chromatography or NMR readouts if the supplier offers them, or check for a certificate of analysis from an independent lab. Establishing a working relationship with suppliers who practice honest quality control instead of paper promises helps cut down on mysteries.
Every bottle of 1-Isopropylimidazole tells a slightly different story. The trick is knowing what’s really in there—not just trusting the label. If everyone upstream and down asks tougher questions about purity, reliability climbs, frustration drops, and fewer discoveries get lost in detective work instead of real science.
Even though a bottle of 1-isopropylimidazole may look harmless tucked away on a shelf, storing chemicals like this at work or in the lab can turn dicey pretty quickly. I’ve handled enough oddball organic intermediates to know mishaps don’t just sneak up in big, sloppy ways—sometimes trouble starts with a cracked cap, a surprise spill, or a forgotten bottle left in a sunny corner. So with 1-isopropylimidazole, careful storage is just plain practical.
1-isopropylimidazole acts as a base, and it can react when mixed with strong acids or certain oxidizing agents. It’ll absorb moisture from the air if the cap isn’t tight, which can change its strength or wreak havoc with sensitive syntheses. The less water that creeps in, the fewer headaches down the line. I’ve worked with similar imidazole derivatives before, and sloppy storage can absolutely throw off your results, with ruined batches and lost time.
Nobody likes a chemical cleanup day, so small habits prevent big problems. I always use tightly sealed glass bottles for this kind of compound—the cheap plastic containers warp, and the threading doesn’t always stand up to repeated use. A good glass bottle with a solid cap goes a long way.
Temperature proves crucial. Room temperature is often fine for 1-isopropylimidazole, as long as things stay steady and cool, away from heat sources and direct sun. Hot spots cause pressure changes in the bottle, which sometimes lead to leaks or vapor buildup. I’ve seen more than one careless person fish out a warped or sticky bottle from a forgotten shelf right above the radiator. That’s asking for a mess.
Moisture plays sneaky tricks in labs. Silica gel packets tossed into a storage cabinet suck away the extra humidity, which keeps the chemical happier, and your risk of fudge-up lower. Dry storage space with little humidity, away from sink splashes and the bathroom door, helps keep things stable. No costly desiccators if you’re strapped for cash—those little silica sachets from shoe boxes work wonders in a jam.
I label bottles clearly, with chemical name, date received, and any hazards. Quick retrieval beats fumbling around with mystery bottles that lost their stickers. Don’t stack everything together, especially acids and oxidizers—shelf segregation isn’t fancy; it’s what keeps drama to a minimum. All it takes is one confused person shelving it next to nitric acid to find out why labels and inventory matter.
Call me old-school, but a safety culture in the lab or warehouse always wins out. Review storage rules with new folks, and don’t just hand them a standard operating procedure with dense text. I’ve hosted short demo sessions, showing exactly how bottles go in the cabinet, which saves dumb mistakes and a ton of unnecessary hassle. Hands-on repetition sticks better than any wall poster.
Nobody signs up for emergency showers or wasted stock. Safe storage of 1-isopropylimidazole comes down to tight seals, dry spaces, and people who pay attention—not a checklist for its own sake, but the difference between a smooth operation and a bottle of regrets.
Chemicals like 1-Isopropylimidazole sneak into lab routines and industrial setups more often than most people realize. It looks tame at first glance—clear, maybe a little silky on the skin. But years working in research labs have shown me that even quiet compounds can throw curveballs. Any chemical from the imidazole family, including this one, can cause skin and eye irritation. Breathe it in, and headaches or a scratchy throat may follow. Some folks feel sick longer after a spill or splash. Risks pile up without attention to details.
Slip on gloves. Not the old pair with holes and stains from past projects, but something designed to hold up to organics: think nitrile or neoprene. Chemical splashes come out of nowhere. Goggles top the list more than once, especially if you deal with powder or liquid forms prone to mist. Standard glasses won't block a spray, and I have seen people blink through the burn of accidental droplets—never fun.
Lab coats help, but shoulders and wrists need coverage, too. Long sleeves stop skin contact in its tracks. These routines sound boring, but worst-case memories—someone coughing all day after a spill or racing to the eye wash—remind me how small habits prevent big disasters.
Good airflow isn't just a bonus. Work with this compound inside a fume hood. It’s tempting to skip the hassle for a “quick mix,” but inhaling vapors—even low amounts—leads to problems. Facilities with broken fans or closed hoods turn every reaction into a riskier bet. Getting used to the hum of fans and smell of filtered air becomes second nature. Smart labs stick ventilation stickers on hoods, making safety checks routine.
Lunch and lab work never mix, not with compounds like this. Eating or storing snacks nearby can lead to accidental cross-contamination. It sounds simple, but people bring bottles or food into workspaces more than you’d think. Old lunch wrappers and coffee cups show chemistry isn’t their top worry in the moment. This habit leads to unintended exposure and, over time, health complaints that seem mysterious but really aren’t.
Store 1-Isopropylimidazole in a dedicated cabinet, away from acids, oxidizers, or anything that likes to cause a reaction. Labels with clear warnings matter more than fancy tracking software; handwritten sticky notes stick with staff memory longer than digital alerts most days.
Accidents don’t wait for a good moment. The clearest voices in the lab are usually ones shouting, “Where’s the eyewash?” Emergency washes and showers need to sit close by, not hidden under piles of storage or behind cardboard boxes. Too many small labs put emergency gear last on the checklist, thinking they’ll never need it. In an accident, hesitation grows with distance. Quick access cuts worry—and cleanup—by half.
Safety isn’t just paperwork. Training sessions, reminders taped above sinks, and casual chats keep everyone sharp. The best learning comes from stories of near misses or what others wish they’d known sooner. I’ve found that sharing these stories helps newbies respect chemicals instead of fearing them. In the end, the real payoff rests with habits built through routine, honest talk, and a lot of double-checking before the first drop ever leaves the container. Safe handling isn’t glamorous, but it helps your shift end with everyone healthy, every single day.
Chemistry can seem like a mess of numbers and letters. For me, though, the molecular formula feels almost personal. It’s a snapshot of what something is at its core. 1-Isopropylimidazole, for example, isn’t a household name. Still, when you spot its formula—C6H10N2—there’s a sense of accuracy in the air. Six carbons, ten hydrogens, two nitrogens. Technically, that’s what you’d find under the microscope. In the lab, people rely on these formulas because any mistake can throw off an entire synthesis, and the recipe falls flat.
The CAS number does something the name can’t. Scientists all over the globe use this set of digits to avoid confusion. Different languages, different brands, no problem—just punch in 3554-74-3 for 1-Isopropylimidazole, and there’s no second-guessing the compound in question. It’s like tracking a package. You might call it “green powder from overseas,” but the tracking number tells you exactly what’s coming, no matter who reads it.
In my college years, I wandered into a lab job where labelling wasn’t always up to snuff. People assumed they could remember what was in each bottle. Guesswork in chemistry means real risk. One slip and suddenly you’ve fused paint to the table or produced nasty fumes. Looking up that CAS number or double-checking a molecular formula saves a lot of headaches. A friend once confused two similar imidazole derivatives because their names were nearly identical. The project ran a week behind schedule, turning a simple oversight into lost funding.
Most folks outside the lab don’t realize how often compounds like 1-Isopropylimidazole weave into everyday products. Some specialty solvents, corrosion inhibitors, and even pharmaceutical intermediates draw on it. Anyone working in these fields depends on precise ingredients. One wrong digit in a formula or a mixed-up CAS, and the final blend flops, sometimes with dangerous side effects. Regulatory checks fall apart without this kind of molecular accuracy. The right data not only protects people but keeps manufacturing running smoothly.
Some think only big corporations care about molecular details. Actually, even small research teams and students need to keep that information handy. Posting CAS numbers and showing molecular formulas on containers helps everyone stay focused and safe. Digital databases like PubChem or ChemSpider make checking these details faster than digging through old textbooks. Barcodes, digital inventory, and clear safety sheets add up to fewer mistakes and stronger lab safety.
Instead of brushing past the numbers on a label, it pays to make double-checking part of the routine. Bringing this habit into classrooms, home labs, and industrial floors can cut down on waste and slapdash science. Plus, students get a sense that details matter early on. With more labs moving toward automation and remote work, locking in the right molecular data has never felt more urgent. Software that crosschecks CAS numbers or flags similar-sounding chemicals can help, but it always comes back to paying close attention.
| Names | |
| Preferred IUPAC name | 1-(Propan-2-yl)-1H-imidazole |
| Other names |
1-Isopropylimidazole 2-Isopropylimidazole Imidazole, 1-(1-methylethyl)- 1-(1-Methylethyl)imidazole |
| Pronunciation | /wʌn aɪsəˈprəʊpɪl ˌɪmɪˈdæzɒl/ |
| Identifiers | |
| CAS Number | [3554-74-3] |
| 3D model (JSmol) | `3DModel:JSmol{"name":"1-Isopropylimidazole","mol":"CN(C)c1cncn1"}` |
| Beilstein Reference | 1209263 |
| ChEBI | CHEBI:139564 |
| ChEMBL | CHEMBL145965 |
| ChemSpider | 160286 |
| DrugBank | DB08733 |
| ECHA InfoCard | ECHA InfoCard: 100.044.283 |
| EC Number | 262-140-2 |
| Gmelin Reference | 82264 |
| KEGG | C21338 |
| MeSH | D007504 |
| PubChem CID | 69735 |
| UNII | 82ZOT32A1E |
| UN number | UN3271 |
| Properties | |
| Chemical formula | C6H10N2 |
| Molar mass | 110.16 g/mol |
| Appearance | White to light yellow crystalline powder |
| Odor | Amine-like |
| Density | 0.987 g/mL |
| Solubility in water | soluble |
| log P | 0.21 |
| Vapor pressure | 0.0295 mmHg at 25 °C |
| Acidity (pKa) | 14.5 |
| Basicity (pKb) | 7.02 |
| Magnetic susceptibility (χ) | -55.5·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.487 |
| Viscosity | 1.39 mPa·s (20°C) |
| Dipole moment | 3.67 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 309.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -15.5 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3795 kJ mol⁻¹ |
| Hazards | |
| Main hazards | Causes severe skin burns and eye damage. Harmful if swallowed. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Hazard statements: "H302, H315, H319, H335 |
| Precautionary statements | P264, P280, P302+P352, P305+P351+P338, P332+P313, P337+P313 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 56°C |
| Autoignition temperature | 530°C |
| Explosive limits | Explosive limits: 1.5–12% |
| Lethal dose or concentration | LD50 (oral, rat): 570 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 > 2000 mg/kg |
| NIOSH | Not established |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 1 mg/L |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Imidazole 2-Methylimidazole 4-Methylimidazole 1-Methylimidazole 1-Ethylimidazole 1-Propylimidazole |