The timeline for discovering imidazole derivatives goes way back to the late nineteenth century. Chemists searching for nitrogen-containing heterocycles bumped into imidazole and realized its potential in pharmaceutical research. After that, tweaking the structure became a bit of a scientific hobby, with researchers adding side groups to unlock new behaviors. Sometime in the late twentieth century, someone put an isobutyl on the first position and a methyl on the second, and 1-isobutyl-2-methylimidazole was born. The journey from obscure lab curiosity to useful intermediate followed a fairly common arc: people tested it, found interesting chemical properties, and began to draft up ways to use it in bulk. The evolution reflects how chemistry advances — slowly, trial by trial, until enough folks see the value in putting resources behind a new compound.
If you walk into any chemical supply catalog, 1-isobutyl-2-methylimidazole appears listed among specialty organic bases and catalysts. Technical staff often seek out such building blocks because they bridge vanilla starting materials and more complex molecules. This imidazole variant teeters between niche and emerging staple, especially in pharmaceutical synthesis where it acts as a route toward functionalized heterocycles. Companies stock it in various purities, usually as a colorless to light yellow liquid, ready to pour into flasks or reactors.
Handling this compound reveals a faint amine-like smell — a little sharp, nothing overwhelming. The boiling point hovers near 260 °C, and its melting point sits well below room temperature, so most find it as a liquid. Solubility leans toward organic solvents, with ethanol and acetone both pulling it into solution easily. Its reactivity stems from the imidazole ring, with electronic characteristics sensitive to both acidity and nucleophiles. The methyl and isobutyl groups skew the overall molecule toward increased lipophilicity, offering chemical versatility that often sees use in both aqueous and organic systems.
Labels for bottles containing 1-isobutyl-2-methylimidazole don’t leave much mystery: CAS number 144919-06-0, molecular formula C8H14N2, and a molecular weight around 138.21 g/mol. Spec sheets typically guarantee a purity greater than 98 percent, with gas chromatography used to verify batches. Manufacturers include basic handling warnings but focus most on proper closure, refrigeration after opening, and containment to avoid vapor concentration in small work spaces. Barcodes and QR codes lead to online safety sheets and analytical data.
In the lab, chemists reach for straightforward methods involving alkylation and cyclization. Start with glyoxal, ammonium acetate, isobutylamine, and methylamine; the process unfolds in several steps, usually involving condensation and ring closure under controlled temperatures. Solvent choice influences yield, with polar options supporting higher selectivity. Upscaling means constant tweaking — anyone making large quantities finds even small changes in pH or reactant concentration tilt the balance between a passable yield and a waste of time. Purification relies mostly on distillation and recrystallization, with column chromatography reserved for particularly sensitive cases.
1-isobutyl-2-methylimidazole puts up with many functional group transformations. The lone nitrogens in the ring invite acylation and alkylation, frequently landing the molecule in multi-step syntheses for drug intermediates. Cross-coupling reactions tack on further complexity. The basicity of the imidazole core makes it a solid base in organometallic reactions or in the formation of metal complexes. Researchers often try to introduce additional substituents on the ring, sometimes blocking positions to steer outcomes when building new bioactive scaffolds.
Chemical registries list it as 1-(2-Methylimidazol-1-yl)-2-methylpropane. Other times, catalogs shorten this to 1-isobutyl-2-methylimidazole or simply IBMI. Sometimes, generic chemical supply codes stand in for full names, but those shopping for it tend to know both the formal and shortcut terms. These synonyms matter in tracking down safety documents and published research, since a single letter in a name can skew a literature search completely off course.
Spills of this liquid need a quick mop-up — the vapor may cause skin or eye irritation, so gloves and goggles keep things from getting uncomfortable. The compound doesn’t ignite easily but does require basic chemical hygiene: avoid direct inhalation, eat nowhere near work surfaces, and store with organics, away from acids. Waste streams containing imidazoles go into designated solvent containers, and standard chemical fume hoods provide enough ventilation during any heating or reaction step.
Pharmaceutical teams lean on 1-isobutyl-2-methylimidazole as a flexible intermediate for building antiviral and antifungal agents. Some teams in polymer research use imidazole derivatives to create specialty resins with unique thermal stability. A few companies have looked into its catalytic properties, as it helps push certain selective methylation reactions further than classic imidazole. The food and fragrance industries pay less attention here, but anyone involved in designing N-heterocycle cores for materials science notices its role in broadening the toolkit for tunable molecular architectures.
Academic teams publish studies aiming to harness structure-activity relationships of imidazole rings, with side groups like isobutyl and methyl offering up playgrounds for binding and selectivity tests. Some efforts direct attention toward green chemistry, with researchers working on solventless synthesis and minimizing hazardous by-products. Recently, collaborative work between medicinal chemistry and computational modeling helps predict how new modifications to the structure might alter potency in potential therapies. Funding agencies now look for both practicality and originality, making it more likely that new routes and applications for derivatives like this will see daylight in published results.
People handling 1-isobutyl-2-methylimidazole rarely report acute toxicity, but data sets for chronic effects still lag behind those for older imidazole variants. Animal studies point to minimal acute toxicity at typical laboratory doses, though any imidazole has the potential for cytotoxic effects at higher concentrations or with long-term exposure. Environmental impact studies suggest the compound breaks down with usual organic matter, but more detailed research on water and soil mobility becomes important, especially in industrial-scale settings. Safe levels for workplace air remain conservative, matching recommendations for similar N-heterocyclic bases.
Many industrial chemists predict growth for 1-isobutyl-2-methylimidazole as new pharma targets call for rare, custom heterocycles. Process improvements for synthesis keep production costs down, while regulatory pressures for green manufacturing challenge teams to drop old, toxic reagents. The next big shift could involve reimagining how this imidazole drives selectivity in both drug and materials synthesis. Open-access data and predictive modeling throw the doors open for unexpected new uses, while the broader scientific community keeps an eye on toxicity findings as the compound moves from bench-top curiosity to more widespread production.
Anyone who’s spent time in a chemistry lab knows molecules like 1-Isobutyl-2-Methylimidazole barely get a sliver of the spotlight, even though they tend to do the heavy lifting. On a typical workday years ago, I sat hunched over a hotplate, shaking a little flask, waiting for something to go right. I’d read about compounds like this one—the quiet enablers—making life easier for people who never see behind the curtain. If you’re curious where this chemical makes a difference, the answer starts with its backbone: the imidazole ring. It’s a structure chemists keep coming back to, mostly because it helps bind, catalyze, and unlock new reactions in ways other families won’t.
Factories and refineries run into trouble with rust—metal just doesn’t last without protection. 1-Isobutyl-2-Methylimidazole gets slipped into corrosion inhibitor blends. I’ve had hands stained by those oily mixtures, helping give pipelines and machinery a fighting chance in places where humidity and chemicals eat metal fast. Companies rely on these imidazole compounds because they grab onto metal surfaces, build up a barrier layer, and slow down rust before it can cause leaks or dangerous breakdowns. Preventing corrosion cuts maintenance costs, but more than that, it keeps operations safer for workers who depend on reliable equipment.
Drug designers love flexibility in their reagents, and this is where the unique shape of 1-Isobutyl-2-Methylimidazole shines. It often steps in when a reaction needs a little nudge—from my experience synthesizing fine chemicals, these compounds can speed things up or steer them in the right direction. In practice, it serves as an intermediate, giving chemists a starting point for building more elaborate molecules. Some antifungals, antihistamines, and even cancer drugs wouldn’t be so easy to make without these stepping stones in the pathway.
Out on the manufacturing floor, making small tweaks to chemical processes can mean less waste, fewer by-products, and higher yields. 1-Isobutyl-2-Methylimidazole brings value here—a predictable performer for both acid-base catalysis and certain cross-coupling reactions. I’ve seen how switching to a tailored imidazole catalyst can chop hours off a batch process or let technicians reduce the amount of heavy solvents. That’s a savings you can measure not just in money, but in fewer barrels of hazardous stuff heading out for disposal.
Not every use for these molecules grows out of pure necessity. Some folks blend them into specialty coatings or advanced plastics because they deliver the kind of thermal and chemical resistance engineers love. Every time a new use gets discussed in our Friday morning meetings, the talk quickly turns to safety, environmental impact, and cost. Disposal can be tricky; imidazoles don’t break down easily, and they may raise eyebrows around the water cooler for potential toxicity in large doses. That’s why it feels urgent to push research toward greener synthesis routes and better recycling. If we’re going to keep these chemicals working for people across all these industries, we owe it to ourselves to do it responsibly—with stronger rules, new filtering tech, and a close eye on how much we actually send downstream.
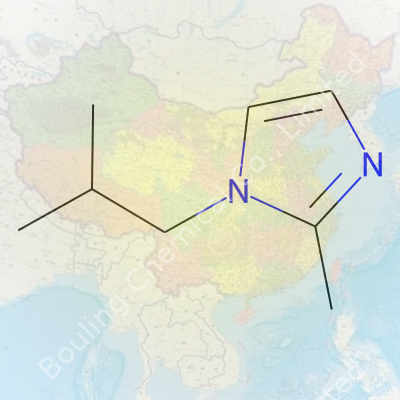
Many folks outside the chemistry lab might hear a name like 1-Isobutyl-2-Methylimidazole and assume it belongs only in textbooks or complex industry reports. It doesn’t. This compound has the formula C8H14N2, which simply means it’s got eight carbon atoms, fourteen hydrogens, and two nitrogens. Fairly compact, definitely not a monster molecule. The CAS number, which serves as a universal fingerprint for every unique chemical, helps avoid mix-ups: 6967-36-0. With this, anyone can look it up in global inventories, from production facilities in Germany to QA labs in Singapore.
I remember the first time I helped a friend trying to source rare compounds for a small research startup. It became clear how even a chemical’s formal identity can make or break logistics, legality, and safe lab practices. Companies, universities, and custom synthesis shops all lean on CAS numbers to keep things tracked and regulated. A mistake in chemical identification can bring research to a halt or even risk accidental exposure. The details in a formula or registry number pull a lot more weight than meets the eye.
Imidazole derivatives like this one aren’t just locked in the realm of academic experimentation. Folks working in specialty polymers, pharmaceutical intermediates, or fine chemical manufacturing bump into these compounds plenty. The methyl and isobutyl bits on the base imidazole change the physical and chemical profile: solubility, volatility, and reactivity all shift, turning an ordinary base material into something fit for targeted jobs. Think of it like tuning a car engine, where a slight tweak can improve performance for one terrain or another.
I’ve seen formulation teams debate late into the evening over which analog to include in a pilot-scale batch. They pore over reaction pathways and yield data, but in reality, it’s often the balance between availability, regulatory status, and handling properties that settles the argument. If 1-Isobutyl-2-Methylimidazole is listed by CAS, and backed by solid safety data, most teams will stick with it rather than roll the dice with something more obscure.
Supply chains for these niche chemicals bring their own headaches. More obscure molecules like 1-Isobutyl-2-Methylimidazole sometimes fall into gray areas. Raw material bottlenecks creep in, as upstream plants focus on high-volume commodities instead. Every extra step before that compound lands on a bench eats into budget and schedule. Some buyers avoid such chemicals, not because they’re unsafe or exotic, but because routine procurement costs too much time or paperwork.
It’s tempting to view a chemical’s formula or CAS as just data, but these tags shape safety protocols, shipping restrictions, and hands-on best practices. A misplaced digit on a safety sheet can trigger audits, or led to incompatible warehousing. Over the years, I’ve seen organizations invest in smarter chemical management tools. They store up-to-date information on clear identifiers, legal restrictions, and vendor data—not just out of compliance, but because it prevents errors that could sideline a whole production run.
Linking research, procurement, and safety databases saves time and cuts down the friction between R&D and purchasing. Clear identifiers, like the CAS for 1-Isobutyl-2-Methylimidazole, give everyone the same reference point—kind of like using a map instead of approximate directions. Building better information bridges saves not only money but headaches, accidents, and unplanned downtime. In my experience, investing in clear lines of communication about these specs always pays off, whether you’re in the lab or running logistics on the factory floor.
You might think proper storage only matters for perishable food, pharmaceuticals, or art. But with this product, a little care translates to better performance, safer use, and longer shelf life. I learned the hard way that skipping out on storage advice turns one small oversight into a ruined batch.
Every product carries its own quirks. Store it somewhere too hot, performance drops. Too cold, it clumps or separates. From what I’ve seen, a dry place with a steady temperature just below room level keeps most products in their prime. Drastic changes can wreck consistency or lead to condensation inside packaging. More than once, I’ve seen decent supplies turn useless after sitting near a radiator or a window.
Moisture invites trouble. Even a pinch of water gets a reaction started before the product ever sees the inside of machinery, kitchen, or lab. I keep my containers tightly shut, especially in humid seasons. One heavy rainstorm or a spilled cup in the wrong spot, and the contents clump beyond saving. A dry cabinet or a shelf far from the laundry room usually does the job. Reinforced double-bagging adds a layer of protection if humidity constantly creeps in.
Rough handling leads to dusty clouds or packaging tears. Small punctures invite air and dampness. I learned that tools matter: metal scoops over hands, and always use clean, dry utensils. If I relied on quick grabs or dirty tools, results suffered. It gets messy, wasteful, and expensive fast.
Some products break down in direct sunlight, turning from useful to wasted in days. Even strong indoor bulbs reduce potency. Cabinets without windows or light-blocking containers give an easy fix. I’ve seen clear canisters lose contents faster than opaque ones. Trust me, the right storage always pays off.
Mixing products together, even unintentionally, creates headaches. One time, a scoop used with flour went into a second bin—big mistake. The flavor, texture, and quality all got weird. I always use separate tools. Wiping off the work area first, labeling containers, and simple routines help sidestep these mistakes.
Freshness counts. Every batch or shipment comes with a date for a reason. Rotating stock so the oldest goes first saves on waste. I check labels every time I open the cabinet. One outdated lot can ruin the next job or recipe. Regular checks save hassle in the long run.
Each link in the chain impacts the next. Manufacturers set the baseline with airtight packaging and clear instructions. Users pick up the baton, storing and handling with care. From lessons I've picked up over years, every shortcut adds risk. Protecting what’s inside the bag or box is a small daily job with outsize payoffs—less waste, better results, fewer costly mishaps.
Working with chemicals isn’t only for people in labs with white coats. A lot of us at some point have handled or been near substances that come with long names and warnings. 1-Isobutyl-2-Methylimidazole falls into that category. This isn’t a household compound like vinegar or baking soda. It tends to show up in research labs and specialty industries, often used as a building block for more complex materials.
Even if you’re no chemist, you’ll want to know why care around this imidazole variety matters. Most of these nitrogen-containing compounds can irritate the skin, eyes, or lungs if something goes wrong. Chemical burns and allergic responses are real risks. The structure pulls in moisture and can travel with vapor or dust. Breathing in fine particles or getting any on your hands can trigger long-term health issues. One slip — no gloves, splash to the eyes, a forgotten mask — that’s how people get burned or wind up at the doctor’s office.
I’ve cleaned up more than a few spills from clumsy mixing jobs or unsteady storage. I remember standing by the laboratory fume hood, watching a co-worker’s glove swell up after a leak in the thumb. Instead of panicking, we moved fast—off with the gloves, lots of water, and straight to the first-aid kit. You learn to respect the gloves, the goggles, and the face shield, not because compliance means a gold star but since nobody wants a trip to the emergency room for a chemical burn.
Take a bottle out of storage on a muggy day and you might notice fumes that sting the nose or throat. Room-level exposure to the vapor can make someone cough or get headaches. Spilled powder will stain skin, sometimes causing rashes or blistering. Like many organic chemicals, it isn’t something you want down the sink or in the trash, either — disposal through municipal water or landfill streams can create headaches for city water managers and pose trouble for wildlife.
Flammability isn’t as obvious as with strong alcohols, but don’t get careless. Sparks and hot heating elements turn careless handling into a fire hazard. In a shop setting, one missed label or shared funnel could lead to a dangerous mix, which nobody wants erupting over a busy benchtop.
Training makes the difference between clean operations and avoidable accidents. Most folks won’t remember the safety data sheet word for word, but everyone can recall a memorable accident or a stern talk from a safety chief. I’ve found that clear labeling, real hands-on drills, and buddy systems turn vague warnings into real muscle memory.
Gloves—never skimp. Chemical-resistant models are a must. Face shields or goggles for splash work stop emergency room visits before they start. Fume hoods eat up vapors and dust and should run before you uncap any bottle. If a spill happens, treat it like a small fire: contain, clean, and never use water unless you know there’s no reaction. Every container needs a clear label with hazard flags in plain English, not just technical codes.
Waste disposal can’t stay an afterthought. Collect solvent or contaminated equipment in a dedicated drum, send it off to certified disposal sites. If you’re training new staff, walk them through the routines, not just with paperwork — let them see a mock clean-up, run a drill, spot-check storage for leaks or poor seals. Safety is less about rules on paper, more about habits people keep every day.
Most folks never hear about 1-Isobutyl-2-Methylimidazole outside of the labs and factories where the real work gets done. For those who handle it, purity isn’t an abstract number but something that actually shapes the end result. The standard most suppliers put forward hovers around 98% or more. Anything lower, and the headaches begin—contamination, failed reactions, and sometimes wasted batches of much more expensive chemicals.
Back in grad school, I ran more than a few experiments where the difference between 97% and 99% purity was the difference between a clean synthesis and a pile of goo. Customers aren’t shelling out for the molecule itself; they’re paying for the peace of mind that they can use it without unexpected surprises. A 1% gap on paper can cost a full day in production loss, or worse, a failed analytical run that forces an entire do-over.
1-Isobutyl-2-Methylimidazole shows up in everything from pharma intermediates to specialty coatings. Trace leftovers from impurities – sometimes solvents, sometimes just unreacted bits from upstream – throw off the next reaction. For people running tight operations, purity above 98% isn’t about chasing a number just for fun. It means you can trust the result, keep records clean, and avoid finger-pointing when batches don’t look right.
Besides purity percentage, suppliers usually spell out water content, color, melting and boiling points, and sometimes specific contaminants that tend to show up based on their process. Low water content matters most for those who can’t tolerate hydrolysis or want to avoid any hint of side reactions. From where I stand, a clear color and a sharp melting point save hours of troubleshooting. Most labs get picky about trace metals or certain organic carryovers, since these tiny bits can play outsized roles in sensitive applications.
I’ve learned the hard way that even high-purity material won’t save the day if the specs dance around between lots. Operators face enough daily uncertainty. Getting a spec sheet that matches batch after batch lets workers focus on the process instead of revalidating every shipment. After all, extra tests eat up time and cash – neither of which appear out of thin air.
The smartest approach isn’t to chase perfect purity, but to ask just what each use needs. Cutting corners slides risk downstream, where it ends up costing more. Responsible suppliers run extra checks and work close with customers willing to pay for tighter specs. On the shop floor, clear communication goes a long way—naming the handful of impurities that matter most, and demanding their minimal presence, brings more value than any high-purity label can on its own.
Laboratory and industrial buyers, in my experience, would benefit from setting well-documented thresholds. Outlining the “must-haves” and the “no-go” contaminants keeps everyone honest. This step isn't glamorous, but nothing about specialty chemicals ever is. What matters most boils down to making sure the next process step unfolds as planned—and purity specs are the closest thing this business has to a safety net.
| Names | |
| Preferred IUPAC name | 2-methyl-1-(2-methylpropyl)-1H-imidazole |
| Other names |
1-Isobutyl-2-methylimidazole 2-Methyl-1-(2-methylpropyl)imidazole |
| Pronunciation | /ˈaɪˌsoʊˌbjuːtɪl tuː ˈmɛθəl ɪˈmɪd.ə.zɔːl/ |
| Identifiers | |
| CAS Number | 69604-68-8 |
| Beilstein Reference | 505416 |
| ChEBI | CHEBI:87184 |
| ChEMBL | CHEMBL3293478 |
| ChemSpider | 19760870 |
| DrugBank | DB08798 |
| ECHA InfoCard | 03c19c8850d7-47a6-8e03-e11d7603bdb1 |
| EC Number | 695-106-0 |
| Gmelin Reference | 148613 |
| KEGG | C19296 |
| MeSH | D015505 |
| PubChem CID | 1266036 |
| RTECS number | NR1575000 |
| UNII | 4V1C8W5I1B |
| UN number | 2810 |
| Properties | |
| Chemical formula | C8H14N2 |
| Molar mass | 138.21 g/mol |
| Appearance | white to light yellow crystalline powder |
| Odor | amine-like |
| Density | 0.97 g/cm3 |
| Solubility in water | Slightly soluble |
| log P | 0.85 |
| Vapor pressure | 0.0197 mmHg at 25°C |
| Acidity (pKa) | 14.5 |
| Basicity (pKb) | 3.46 |
| Magnetic susceptibility (χ) | -62.7×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.493 |
| Viscosity | 3.2 mPa·s (25 °C) |
| Dipole moment | 2.94 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 359.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -4906 kJ·mol⁻¹ |
| Hazards | |
| Main hazards | Causes severe skin burns and eye damage. Causes serious eye damage. Harmful if swallowed. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P280, P305+P351+P338, P337+P313, P261, P304+P340, P312 |
| Flash point | Flash point: 112°C |
| Autoignition temperature | 355°C |
| Lethal dose or concentration | LD50 (oral, rat): 1180 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 1300 mg/kg |
| NIOSH | UT3604000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 1-Isobutyl-2-Methylimidazole: Not established |
| REL (Recommended) | 0.05 ppm |
| Related compounds | |
| Related compounds |
1-Butyl-2-Methylimidazole 1-Isopropyl-2-Methylimidazole 1-Methyl-2-Methylimidazole 2-Methylimidazole 1-Ethyl-2-Methylimidazole |