Chemistry often moves forward on the shoulders of bold syntheses, and 1-Iodopyrrolidine-2,5-dione is no exception. Research circles first became aware of the potential for N-haloimides in the twentieth century, as organic synthesis sought milder reagents with selectivity for halogenation. The story picks up with the quest for novel iodinating agents distinct from elemental iodine and hydrogen iodide. As far back as the 1960s, key papers detailed the development of N-iodosuccinimide, offering organic chemists a tool that reduces byproduct formation in sensitive syntheses. Soon, the spotlight spread to derivatives like 1-Iodopyrrolidine-2,5-dione, allowing more customized reactivity and making the compound a staple in laboratories focused on selective iodination.
1-Iodopyrrolidine-2,5-dione presents as a versatile iodinating agent where pyrrolidine rings replace succinimide’s more planar structure. The nitrogen atom supports iodine in an electron-rich framework, lending the compound distinct reactivity among N-haloimides. Laboratories benefit from handling a crystalline solid, easy to weigh and store, with lower volatility than many molecular iodine sources. Its reliable liberation of iodine in both electrophilic and radical reactions keeps it on the shelves of organic, pharmaceutical, and material chemists alike. For synthetic paths that demand mild but strong iodination, this chemical has earned a spot as a reagent of choice.
1-Iodopyrrolidine-2,5-dione carries the molecular formula C4H4INO2 and a molar mass that rests just over 241 grams per mole. Most chemists recognize it by its distinct crystalline structure, often white to off-white in color, with sufficient stability for benchtop storage under well-sealed containers. This solid resists deliquescence, even in moderately humid labs. It dissolves well in common organic solvents, including dichloromethane, chloroform, and sometimes acetonitrile, which makes it handy for diverse reaction setups. The molecule itself holds an iodine atom bound at a nitrogen, offering latent reactivity; this position encourages donation during reactions such as iodination of alkenes or aromatics.
Precision is essential. Most suppliers list guaranteed purities above 98% for 1-Iodopyrrolidine-2,5-dione, minimizing batch-to-batch variability. Labels typically identify hazard classes per GHS standards, emphasizing the oxidizing potential and need for dry, cool storage. Key technical data—melting point, solubility profile, batch number, expiry, and synthesis route—usually appears on the label or the associated technical sheet. Labs tracking compliance with REACH or other environmental standards demand full disclosure of supply chain and impurity profile, which only reputable producers can provide reliably.
The go-to preparation route involves oxidative iodination. Most chemists start from pyrrolidine-2,5-dione and introduce elemental iodine with an oxidizing agent such as potassium iodate, sodium hypochlorite, or similar. The process includes careful control of temperature and solvent choice. Chlorinated solvents prove popular for their stabilization, but increasing attention shifts toward greener media as environmental consciousness grows. Upon reaction completion, excess reagents are neutralized before isolation of the solid product, often by filtration from the cold reaction mixture. Recrystallization from compatible solvents yields a product with high purity, suitable for demanding research applications.
In synthetic chemistry, 1-Iodopyrrolidine-2,5-dione stands out for transferring iodine atoms to electron-rich substrates. This makes it particularly useful in aromatic iodination, furnishing halogenated intermediates valuable for cross-coupling reactions. The molecule also finds use in activating alkynes and alkenes, facilitating electrophilic addition of iodine to generate vicinal diiodides. Beyond direct iodination, creative chemists use it in tandem with transition metals to initiate cascade transformations—expanding the synthetic toolkit for complex molecule construction. By altering substituents on the pyrrolidine ring, researchers can sometimes tune the release profile, opening new doors for controlled reactivity or site-specific modifications.
While 1-Iodopyrrolidine-2,5-dione is the IUPAC preferred name, chemical catalogs and academic texts regularly use trade names and abbreviations for shorthand. References to N-Iodopyrrolidone, N-Iodopyrrolidin-2,5-dione, or even “IPD” are common. Sometimes, confusion arises because close relatives—such as N-iodosuccinimide—occupy similar environments. For supply chain transparency, reference to the CAS number (usually 89030-92-6) ensures accurate communication across vendors and regulatory bodies.
Anyone who’s worked with halogenating agents knows the respect they command. 1-Iodopyrrolidine-2,5-dione releases iodine easily, so skin contact and inhalation present clear hazards. Labs operating with this compound enforce stringent PPE guidelines: gloves, goggles, and fume hoods. Material Safety Data Sheets stress that heating or mixing with strong reducing agents risks violent decomposition. Waste treatment procedures must address not just organic material but potential iodine contamination, often involving reduction to less hazardous iodide before disposal. Regular training and periodic review of safety protocols ensure the prevention of incidents, which matters as regulatory scrutiny on hazardous reagents continues to rise worldwide.
The true value of 1-Iodopyrrolidine-2,5-dione shows up in how often researchers reach for it during synthesis planning. Medicinal chemists use it to prepare radio-iodinated tracers for diagnostic imaging. Material scientists deploy it for surface activation prior to polymer modification. Its role in synthesizing aryl iodides sets the stage for Suzuki and Sonogashira cross-couplings, powerful tools in drug development. The academic lab benefits from robust selectivity in iodination of both electron-rich and moderately activated substrates, which advances the exploration of reaction mechanisms and structure-activity relationships in small molecule research.
Continuous research into 1-Iodopyrrolidine-2,5-dione pushes boundaries in reaction scope and efficiency. Academic groups focus on greener synthesis routes to minimize environmental impact; solventless and aqueous-phase processes have moved from curiosity to practical reality. Industrial innovation zeroes in on process intensification—flow chemistry setups and in-line monitoring reduce reagent use and waste. Meanwhile, studies of reagent tuning seek to lock in selectivity for specific transformations, where minute electronic changes to the pyrrolidine ring shift the reagent’s behavior in valuable ways. Collaboration between chemical manufacturers and end users sparks feedback that keeps new advances grounded in actual laboratory needs.
Detailed studies show 1-Iodopyrrolidine-2,5-dione’s risk profile is similar to related N-haloimides. Moderate toxicity to aquatic life means responsible disposal is non-negotiable; laboratory staff stay updated on environmental fate to ensure compliance. Chronic exposure data remains limited, so best practices lean toward caution. Inhalation and ingestion studies in rodents show dose-dependent effects mainly tied to iodine’s inherent toxicity rather than the pyrrolidinedione backbone. Applying lessons from related compounds, scientists advance in vitro assays to anticipate risks before scaling up to industrial operations. Vigilance in toxicity screening will keep workers and the wider community safe as uses expand.
The next few years will likely see 1-Iodopyrrolidine-2,5-dione shift from specialty reagent to more mainstream synthetic tool. As pharma and agricultural companies hunt for better ways to install iodine into complex molecules, demand grows for reagents that balance reactivity, selectivity, and manageable safety profiles. Ongoing education in green chemistry, supported by detailed mechanistic studies, lays a solid foundation for further innovation. By bridging classic reagent chemistry with emerging demands for sustainability, this compound looks set to remain a favorite for a new generation of synthetic challenges—proving that, even as tools evolve, fundamental chemistry continues to drive discovery and application both in the lab and in industry.
1-Iodopyrrolidine-2,5-dione stands out among chemical reagents, recognized more widely in labs as N-Iodosuccinimide (NIS). This compound often pops up in organic synthesis, offering researchers a reliable source of electrophilic iodine. Chemists lean on NIS when they want to add iodine atoms to molecules without bringing the chaos of elemental iodine into the mix. Precision matters in these reactions. Small changes at the molecular level can flip the script on a drug’s activity or help build a more effective material.
NIS rarely jumps into the spotlight, but its mark appears in a handful of useful transformations. In my own lab experience, recipes that call for iodination often turn to NIS for its ability to deliver iodine gently. That means it can attach an iodine atom to a benzene ring, or tweak delicate molecules, without ripping them apart. This kind of control opens new doors in pharmaceuticals and crop science. Iodine can turn a bland molecule into something that targets a virus or boosts crop yields. Real examples include synthesizing antiviral drugs or switching up the building blocks for plant protectants.
Simplicity on the surface rarely matches the complexity below. NIS seems simple, yet it has made itself almost indispensable. Drug developers use it to make molecules more amenable to further tweaking. Medicinal chemistry thrives on these small changes. When one iodine atom lands in the right spot, it affects how a drug travels in the body or how long it sticks around to deliver its effect.
Outside medicine, NIS also enters the conversation for materials science. For example, it helps add the right functional groups to polymers. This process shapes how plastics behave or lets them grab onto metals or dyes. The consumer never sees NIS, but its fingerprints show up on products ranging from electronics to coatings.
Every responsible chemist weighs the safety question. Handling NIS demands gloves and goggles since it can cause burns and eye damage. It doesn't work well with water and leaves behind iodine-containing byproducts. If those slip through the cracks, they damage aquatic life and mix up the ecosystem in ways we don’t always predict. Labs treating their waste before disposal keep this in check, but wide industrial use multiplies the need for oversight. Regulations continue to evolve as scientists learn more about what's leaving their benches and where it travels downstream.
Green chemistry attracts plenty of headlines, but behind closed doors, researchers look for kinder reagents every year. Some have tried switching to more benign iodine sources or cutting the use of halogenated chemicals. In practice, finding that perfect substitute for NIS takes stubbornness and creativity. It comes down to trade-offs: performance, cost, and how well a replacement fits the process. Developing iodine sources that skip over the health and waste issues, yet do the chemistry cleanly, stands as a frontier worth crossing. Until then, NIS will keep showing up in the daily grind of building smarter drugs and better materials.
Picture the pyrrolidine ring, a five-membered structure so common in organic chemistry, and now imagine slapping an iodine atom at the one position. Now toss on two carbonyl groups at positions two and five, and that’s the backbone of 1-Iodopyrrolidine-2,5-Dione. The chemical formula spells out as C4H4INO2. It’s always rewarding to nail down a structure so cleanly in your head — especially after seeing how a single atom swap, like dropping in an iodine, shifts the behavior and even the color of the whole compound. That iodine is a beast, carrying real heft compared to the lithe hydrogens it often replaces.
Numbers turn abstract chemistry into something concrete you can measure, weigh, and trust in the lab. The molecular weight for 1-Iodopyrrolidine-2,5-Dione adds up like this:
Total: 48.04 + 4.04 + 126.90 + 14.01 + 32.00 = 224.99 g/mol
That’s a big molecular weight for such a small ring, showing just how much one halogen can tip the scale. Out of all the halogens, iodine gives the most dramatic jump — it’s why you feel it in everything from cost per gram to regulatory paperwork. In chemical industries, this figure guides batch preparation, pricing, and even safety sheets.
Knowing the exact formula and molecular weight isn’t a trick for textbooks or an exercise for passing exams. In my lab days, getting this wrong meant trashed samples or inaccurate results. It hits harder in manufacturing, where incorrect weights can lead to batch failures, safety incidents, or regulatory headaches. Labs reach for these numbers day in and day out — making buffers, planning syntheses, writing safety data sheets. Agencies like OSHA and the EPA rely on these values to advise on handling and safe exposure limits. Getting it right means fewer surprises when scaling up experiments or moving from the bench to the plant floor.
It’s not just scientists who care, though. Regulatory bodies, procurement managers, and even university students tap into these data points. Global standardization lets researchers from different countries compare notes, share results, and reproduce work. You get trust and progress, not just in a single lab but on an international scale. Robust information about small molecules like 1-Iodopyrrolidine-2,5-Dione acts as the foundation for safer research and smoother business.
Information about small molecules floats around, but not always accessibly. Public databases, open-access journals, and government sites should keep the details available without hidden paywalls or registration. Thorough peer review and community feedback, especially in high-traffic repositories like PubChem or ChemSpider, help catch mistakes early. Vendors and educators need to pull from these trusted pools, not isolated spreadsheets. It’s time for every classroom and workplace lab to have an updated, reliable toolkit that goes beyond a dusty data book.
Anybody working in a lab learns quickly that chemicals are finicky houseguests. 1-Iodopyrrolidine-2,5-dione certainly falls into that world—reactive, moisture-sensitive, and not forgiving if treated like a jar of sugar. As someone who's handled chemicals from freshman year to professional life, ignoring good storage practices can end with ruined experiments or worse. So, this isn’t about red tape. It’s about making sure nothing goes wrong, health stays intact, and research money doesn’t fly out the window.
Moisture sets off 1-Iodopyrrolidine-2,5-dione easily, breaking it down and making it useless or unsafe. Any open container left near a sink or wash station? It draws in water from the air much faster than you'd think. I've seen someone shrug off recapping, leave a bottle in a humid room, and find a sticky mess with extra byproducts the next morning. The right practice means tight caps and sealed containers—preferably in a desiccator or with packets of drying agent right inside the storage box.
Sunlight and heat give unpredictable results. Most labs use refrigerators, but temperature swings from opened doors or an overfilled unit aren’t ideal. That’s why placing this compound in a labeled, dedicated, low-traffic fridge shelf keeps things steady. Posting a quick note with the chemical’s sensitivities helps keep new staff from making basic mistakes.
There’s no such thing as too much precaution in chemical storage. I can’t count how often someone in a crowded lab bumped a shelf or dropped a bottle on the way to weighing. Any glass container should stay in a secondary plastic tray—those trays catch leaks, making cleanup ten times easier.
Chelating agents or reducing agents kept near iodine-based compounds can spark off dangerous reactions. Never store 1-Iodopyrrolidine-2,5-dione near strong acids, bases, or materials that might ignite. I learned this the hard way after a close call in a university storeroom: a misplaced oxidizer next to some acetone almost set off a chain reaction during summer break. Good labeling, regular shelf checks, and a chemical inventory system are real life savers—not just checkboxes for compliance.
Letting everyone grab what they want from storage cages or rooms leads to accidents, theft or untraceable spillage. In a professional setting, trusted staff keep track of who takes what and when. It does slow down a day, but that layer of accountability keeps everyone honest and helps track expiration dates before anything goes stale or dangerous.
Chemicals like this don’t just disappear. Unused or decomposed material needs professional waste handling. Following your institution’s hazardous waste process might feel slow, but cutting corners lands labs with fines, contamination, or even hospital visits. Anyone who’s had to clean up spilled iodine will say it’s better not to tempt fate.
Every detail in storing 1-Iodopyrrolidine-2,5-dione plays a role in scientific integrity, personal safety, and real cost savings. Dry, cool, clearly labeled, and well-accounted storage always turns out to be worth the extra steps. Most laboratory mishaps I’ve seen have less to do with complicated procedures, and more with someone thinking, “That’ll be fine, just this once.” Trust experience: It usually isn’t.
1-Iodopyrrolidine-2,5-dione isn’t something most people talk about over coffee. Chemists know it better as N-iodosuccinimide, a reagent that shows up in labs and in chemical synthesis. If this compound shows up near where people work or study, questions pop up fast: “Is it safe?” “What can go wrong?” Anyone handling unfamiliar chemicals wonders about toxicity. Having spent years in both research and teaching, I’ve learned to respect these questions, since the smallest oversight can bring big trouble.
N-iodosuccinimide breaks down into iodine and succinimide. Neither bit seems so menacing on paper. Yet once chemical reactions start, byproducts gain new properties. This iodine-based compound reacts strongly with a range of organic chemicals, causing it to be categorized among strong oxidizing agents. Those can do damage if people don’t follow strict controls.
Handling it without gloves causes skin irritation. Inhaling dust or vapors may bother the nose and lungs. The eyes are especially vulnerable; accidental splashes make them burn, water, and swell. People with existing respiratory sensitivities could be more affected, especially in poorly ventilated rooms. Chronic exposure might ramp up these risks.
Lab incident reports back up these concerns. I once watched a careless graduate student skip proper protection, hoping to “just measure a little” without gloves. He left with red, itchy hands and a new respect for the warning labels. The data lines up: the Globally Harmonized System (GHS) marks N-iodosuccinimide as an irritant and an environmental hazard, and advises minimizing exposure.
Nobody expects instant disaster from trace amounts, but high concentrations leave damage. Succinimide derivatives linked with improper disposal have shown toxic effects in aquatic organisms, which means labs and manufacturers must carefully manage waste. Iodine compounds also disrupt some microbial systems, making them problematic for water treatment facilities.
Scientists and safety teams keep watch over how much and how often these substances enter local water and soil. In research settings, the push has been toward green chemistry—reducing not just immediate harm, but secondary environmental fallout. Substituting less hazardous chemicals, scaling down use, and using closed-system transfers help to tamp down risks.
Protection starts simple: gloves, goggles, fume hoods, and training. Moving the bottle from shelf to scale inside a controlled area, with a full spill kit nearby, matters more than memorizing any technical sheet. Anyone who works with young lab members or new hires picks up on this quickly, because new mistakes happen not from a lack of intelligence, but from rushing or poor communication.
Good labeling, up-to-date Safety Data Sheets (SDS), and management buy-in all shape how this chemical gets handled. Periodic training helps even veterans remember what’s at stake. Labs that handle N-iodosuccinimide regularly have written protocols for cleanup, emergency showers, and disposal, so nothing is left to chance.
Reducing hazard comes from honest conversations about risk and practical steps. Alternatives exist for some lab uses, giving chemists safer routes to the same results. Where substitution isn’t possible, investment in protective gear, engineering controls, and steady education brings down the odds of harm. Rushed work or shortcuts usually set the stage for problems; taking the time to do things safely protects both people and the environment.
Chemists talk a lot about solubility, but most people never give it a second thought. In research labs, 1-Iodopyrrolidine-2,5-dione isn’t just another name on a bottle. Its behavior in different solvents sparks plenty of curiosity, especially among those working in pharmaceutical synthesis or advanced organic chemistry. The solubility of this compound can make or break certain reactions, so understanding it isn’t just a nerdy detail—it’s critical.
If you drop some 1-Iodopyrrolidine-2,5-dione into water, you don’t see much happen. This compound holds a decent level of polarity thanks to the imide structure, yet the bulky iodine atom adds non-polar character. Water, stubborn as always, doesn’t let it in easily. The crystals mostly hang around at the bottom, hinting at low but not zero solubility.
The real action shows up in organic solvents. I remember tossing it into acetone during a synthesis and seeing it vanish almost instantly. Acetonitrile gives a similar result. Both solvents balance polarity and are excellent for dissolving compounds that can’t decide if they want to play nice with polar or non-polar media. In my own work, this versatility smoothed plenty of rough patches during multi-step reactions.
Try alcohols, and you’ll see mixed results. Methanol handles it fairly well, likely because its small size and polarity catch the imide part, but ethanol starts to struggle. Chloroform and dichloromethane, both non-polar, pull it in nicely. This trait matters in extraction procedures: you can partition the compound from water into a halogenated solvent with less fuss, streamlining purification.
Putting any halogenated imide into solution brings safety into the conversation. 1-Iodopyrrolidine-2,5-dione isn’t only reactive—it’s also a strong oxidizer. Knowledge about its solubility guides proper handling because solutions turn more dangerous as concentrations rise. Acetone and chloroform dissolved it far faster for me, but that also meant higher risk if spills or splashes happened.
Facts from chemical safety data sheets back this up. A bigger dissolved load means stronger oxidizer action in the liquid phase. Spills need immediate attention, not just for chemical burns but for fire risk too. Fume hoods and gloves aren’t just protocol—they keep you in the game.
Where solubility lands, waste disposal questions soon follow. Strong dissolving power in chlorinated solvents translates into sticky residues and tricky clean-up. My own bench always needed extra effort after using DCM or chloroform. Labs should remember: the easier 1-Iodopyrrolidine-2,5-dione dissolves, the tougher it gets to clean up responsibly.
Green chemistry strategies push researchers toward less harmful solvents. I’ve watched colleagues switch out DCM for ethyl acetate, sometimes trading off solubility for safety. Newer solvent mixes offer middle ground, but the transition demands more than just swapping bottles—it involves testing, tweaking, and training. Chemical R&D budgets rarely stretch enough, so everyone gets creative with what’s on hand.
Solubility shapes real decisions by anyone using 1-Iodopyrrolidine-2,5-dione. Good habits—double-checking which solvent to use, working under a hood, keeping chemical inventory fresh—grow from knowing these properties. Junior chemists in the lab pick this up quickly, usually after seeing someone else wrestle with an unexpected spill or failed separation. Shared knowledge beats reading tables alone.
As research grows more complicated, so do the questions around specialty reagents and their behavior in the lab. Solubility might look like a dry, technical detail, but for those mixing, pouring, and cleaning up, it makes a big difference. Smart solvent use builds safer protocols, shapes more efficient reactions, and trims environmental impact. That’s real progress, one measured scoop at a time.
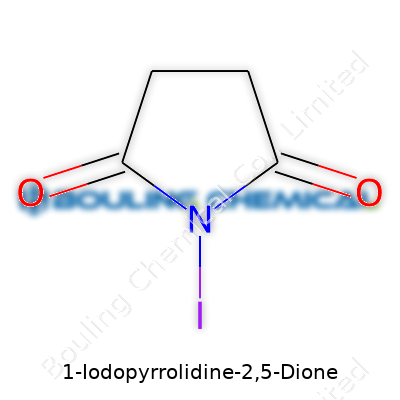
| Names | |
| Preferred IUPAC name | 1-iodopyrrolidine-2,5-dione |
| Other names |
N-Iodosuccinimide NIS 1-Iodosuccinimide |
| Pronunciation | /ˈaɪ.oʊdoʊ.pɪˌroʊ.lɪˌdiːn ˌtuː.faɪv ˈdaɪ.oʊn/ |
| Identifiers | |
| CAS Number | 1533-82-6 |
| 3D model (JSmol) | 3D model (JSmol) string for **1-Iodopyrrolidine-2,5-dione**: ``` IO=C1CC(=O)N1 ``` |
| Beilstein Reference | 88828 |
| ChEBI | CHEBI:156689 |
| ChEMBL | CHEMBL128942 |
| ChemSpider | 13280835 |
| DrugBank | DB08339 |
| ECHA InfoCard | 04c94e38-adac-473b-9871-84d94c8bd53c |
| EC Number | 253-025-4 |
| Gmelin Reference | 1629611 |
| KEGG | C14330 |
| MeSH | D000319 |
| PubChem CID | 69998738 |
| RTECS number | UX8225000 |
| UNII | 21P3BP7PDT |
| UN number | Not regulated |
| CompTox Dashboard (EPA) | urn:CST:3bbcf79f-cad6-4f5e-8dc3-c5763c08ea4e |
| Properties | |
| Chemical formula | C4H4INO2 |
| Molar mass | Molar mass: 223.98 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.904 g/cm³ |
| Solubility in water | slightly soluble |
| log P | -0.2 |
| Vapor pressure | 0.000244 mmHg at 25°C |
| Acidity (pKa) | 8.5 |
| Basicity (pKb) | -1.42 |
| Magnetic susceptibility (χ) | -75.0e-6 cm³/mol |
| Refractive index (nD) | 1.721 |
| Viscosity | Viscous liquid |
| Dipole moment | 4.75 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 333.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -107.0 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -2114 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin irritation, causes serious eye irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | Health: 2, Flammability: 1, Instability: 1, Special: |
| Flash point | 104.5 °C |
| LD50 (median dose) | LD50 (median dose) of 1-Iodopyrrolidine-2,5-Dione: 640 mg/kg (oral, mouse) |
| NIOSH | WH6650000 |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 0.02 ppm |
| IDLH (Immediate danger) | NIOSH: Unknown |
| Related compounds | |
| Related compounds |
N-Iodosuccinimide Succinimide N-Bromosuccinimide N-Chlorosuccinimide Pyrrolidine-2,5-dione |