Chemists in the mid-20th century began taking a closer look at pyrrolidine derivatives, searching for amines with new uses in medicine, agriculture, and material science. 1-Ethylpyrrolidin-2-Ylmethylamine attracted attention thanks to its balance of reactivity and stability, fitting nicely into synthetic schemes but not breaking down at the first sign of humidity or heat. Companies followed academic leads, refining synthesis routes and slowly unlocking the compound’s potential. By the 1980s, this molecule often showed up in research journals and patent filings, not as a blockbuster product but as a workhorse intermediate — the sort chemists rely on when chasing novel targets in drug or material development. The story of this amine echoes a lot of quiet advancements in chemistry: incremental breakthroughs driven by patient experimentation, industry feedback, and problem-solving more than marketing hype.
1-Ethylpyrrolidin-2-Ylmethylamine provides a convenient nitrogen source with structural versatility. The ethyl group on the pyrrolidine ring influences its electronic properties and its interaction with other molecules. Sellers usually offer it in small, sealed containers, labeling it as a research chemical, though specialists in pharmaceuticals, agrochemicals, and polymers know it as a reliable reagent for further synthesis. Chemists using this amine value its reactivity, but also its manageable storage and transport needs—attributes vital for both bench research and scale-up.
This compound shows up as a colorless to pale yellow liquid with a slight amine odor. Its molecular weight lands in the moderate range for amines, and it dissolves in polar solvents including methanol, ethanol, and water, a helpful property for multi-step synthesis. The secondary amine tail brings nucleophilic character, making it participate in amide, imine, and reductive amination chemistry with little fuss. It tends not to oxidize rapidly in air, and standard refrigeration extends shelf-life. Boiling point hovers around 200-220°C, which makes it easier to handle than many low-boiling amines. In terms of safety, its vapor pressure stays low enough to keep inhalation risks modest if good ventilation is present.
Specifications for 1-ethylpyrrolidin-2-ylmethylamine can differ from one supplier to another, but the essentials don’t change much. Purity typically exceeds 97%, and many labs call for HPLC or GC trace analysis to double-check for residual solvents and byproducts. Labels should list lot number, gross and net weight, manufacturing date, expiration date, recommended storage temperature, and clear hazard warnings. Strict attention to labeling ensures traceability—important if problems crop up downstream in synthesis or if a batch turns out off-spec after shipping. Clear information helps everyone from warehouse staff to R&D chemists avoid costly mistakes or unsafe conditions.
Chemical manufacturers often opt for alkylation of pyrrolidin-2-ylmethylamine with an ethyl halide under basic conditions. Sometimes, they rely on reductive amination where pyrrolidin-2-one reacts with a suitable aldehyde, followed by reduction, to get to the targeted structure. The choice depends on cost, yield, and the nature of side products. Small-scale research settings generally use the simplest, reproducible methods, while plant-scale operations optimize for throughput, waste minimization, and ease of purity control. Process teams continually tinker with solvents, catalysts, and purification steps as part of quality assurance efforts, especially since even minor contamination affects downstream applications in pharma or electronics.
The secondary amine group lets chemists install acyl, alkyl, or aromatic moieties to build diverse compounds. Amidation and reductive amination both work well, and protection of the nitrogen is straightforward with standard protective groups. Electrophilic aromatic substitutions proceed at predictable spots thanks to electron donation from the nitrogen. Cross-coupling partners sometimes get attached via palladium or copper catalysts, turning this simple amine into a starting material for more elaborate molecular scaffolds. Even small changes to ring or side-chain structure in this family of compounds tend to trigger variations in activity and physical properties, drawing attention from drug designers and materials scientists alike.
This molecule often appears under alternative names in catalogs, patents, or journals. Some call it 1-ethyl-2-pyrrolidinylmethylamine, others might shorten it to N-ethylpyrrolidin-2-ylmethylamine. IUPAC naming ensures clarity, but sales channels sometimes favor simpler or legacy nomenclature, adding a wrinkle for those searching across different databases. In older European literature or Asian product listings, translation quirks or batch codes sometimes create confusion. Consistent use of CAS numbers helps reduce errors, but every research chemist I know keeps a thumb on the cross-references, just to avoid ordering the wrong bottle in a rush.
Handling of 1-ethylpyrrolidin-2-ylmethylamine means working with gloves, safety goggles, good ventilation, and strict attention to hygiene. The liquid can cause skin, eye, and respiratory tract irritation, so workspace protocols matter. Manufacturers provide material safety data sheets spelling out proper disposal, spill response, and fire precautions. Storage means cool, dry, tightly capped containers, away from incompatible acids or oxidizers. Personnel learn through training or hard-won experience that respect for these routines makes for fewer surprises in the lab. Spills or splashes rarely happen if people follow the basics, but an eye-wash station within reach remains non-negotiable. In production settings, fume hoods or local exhaust keep exposure levels very low, and engineering controls routinely get checked.
This molecule doesn’t sit on the shelf for long in active chemistry labs. Drug developers tap it as a precursor to alkaloid analogs, CNS-active agents, and a variety of lead compounds aimed at neurological or cardiovascular targets. The agricultural chemical sector exploits its amine portion as a building block for herbicides and plant growth regulators. Material scientists look to it as a linker, crosslinker, or as a functional group for advanced polymers and specialty adhesives. Each of these applications springs from the ability to further modify the core structure quickly and economically. Even outside these focused industries, synthetic chemists often add it to their toolbox for installing secondary amine linkage or exploring new chemical space in structure-activity relationship studies.
New developments in pyrrolidine chemistry often tap into molecules like 1-ethylpyrrolidin-2-ylmethylamine. Pharmaceutical innovators scan for modifications that give better solubility, metabolic stability, or selectivity, starting with robust scaffolds like this. Recent projects in my network experimented with changing the ethyl tail for bulkier groups, hoping for new effects without the risk of flagging toxicology alerts. Teams in green chemistry experiment with more sustainable solvents or biocatalytic routes to bring down environmental impact, aiming to replace traditional base-catalyzed reactions with enzyme-based methods or recyclable catalysts. Universities and start-ups swap insights at conferences about optimizing yields, handling, and new reactions, turning this modest amine into a springboard for broader innovation.
Toxicology teams focus on skin and eye sensitization, acute inhalation hazard, and metabolic fate for synthetic amines. Early screens indicate moderate irritant potential, with care needed during transfer and dilution. In animal models, metabolism tends to favor oxidation and excretion, but some derivatives build up in tissues, pushing for close monitoring and further study. Long-term effects in humans remain uncertain, which steers responsible users toward tight containment and strong ventilation. Regulatory reviews sometimes flag certain pyrrolidine-based compounds for detailed assessment, especially if structure-activity relationships hint at reactivity towards proteins or DNA. Access to up-to-date safety findings, coupled with real-life lab experience, keeps risk low but ever-present on everyone’s checklist.
As drug design and materials science keep hunting for specialty amines, 1-ethylpyrrolidin-2-ylmethylamine should hold its ground as a key intermediate. Advances in biocatalysis and automation might cut costs and environmental risks, turning this molecule even more accessible for industry and academia. In my view, one challenge will always be the push for safer, greener synthesis with tighter control over side products. Regulatory pressure in Europe, North America, and Asia means every producer and user has to document traceability and safety throughout the supply chain. Makers who invest in such oversight will carve out an edge, especially as customers demand transparency. Although new amines and analogs constantly hit the market, few offer the mix of reliability, modifiability, and manageable risk seen here. Steady improvements in manufacturing, purification, and downstream chemistry keep this compound right in the thick of chemical R&D — a quiet testament to the enduring power of iterative progress in the lab and on the factory floor.
1-Ethylpyrrolidin-2-ylmethylamine isn’t the sort of molecule most people run into daily, but for those who’ve spent time in pharmaceutical or chemical research, its name pops up more than you’d think. It belongs to a wide family of compounds researchers rely on for making new molecules, especially those aimed at drug development. Labs focus on this kind of building block for one reason: it helps create complex structures faster and with fewer headaches. Instead of slogging through a dozen reaction steps, chemists can slot this amine in and push forward with a targeted synthesis.
Drug discovery works a bit like solving a thousands-piece puzzle without the picture on the box. 1-Ethylpyrrolidin-2-ylmethylamine gives chemists a sturdy piece for their puzzle. It brings a pyrrolidine ring, which frequently shows up in medicines that act on the nervous system. You see similar compounds in antiviral drugs, anti-psychotics, and pain medication candidates. The presence of this ring system in active drugs means researchers keep coming back to it when they look for something new that tweaks biological pathways in helpful ways.
Trying to build new drugs means juggling cost, time, and safety. Every shortcut that lets a researcher cut down the number of steps saves not just money but a lot of wasted work. From my time on a team looking to synthesize neuroactive molecules, having an amine like this in the freezer meant we dodged several nights camped next to a reflux setup. The extra ethyl group opens up different chemical reactions. Usually, these tweaks allow scientists to produce compounds that fit into a protein target more snugly—or to avoid breaking down too fast in the body. These details can spell the difference between another failed project and something that makes it into animal studies.
While making new drugs carries lots of promise, there’s always a darker side. Certain pyrrolidine-based amines have drawn the attention of regulatory agencies due to their potential for misuse or for showing up in controlled substances. Synthetic chemists have stories of new molecules with unexpected effects turning up on the market in unsupervised ways. Even a single tweak sometimes leads to a compound that mimics pharmaceuticals far too closely. This is where clear guidelines and oversight keep things honest and safe. Chemical suppliers register sales, and regulators ask questions at every step of the process.
A path forward involves balancing the need for innovation against public safety. Universities and research labs train new chemists not just in making molecules efficiently but also in responsible handling. There’s a lot of paperwork behind every bottle of specialty amine. Sharing knowledge helps too; when chemists publish details on how these chemicals interact with biological systems, the community stays alert for problems. Open forums and peer-review keep everyone on their toes, building trust while allowing new medicines to reach people who need them.
No one wants to slow down the search for new treatments. At the same time, the line between potential therapy and potential hazard can get thin. People with direct lab experience know the routine: logging chemicals in and out, sticking to protocols, working with colleagues who spot trouble before it starts. This kind of culture builds a safer and smarter environment not just for the researchers but for everyone who depends on the results.
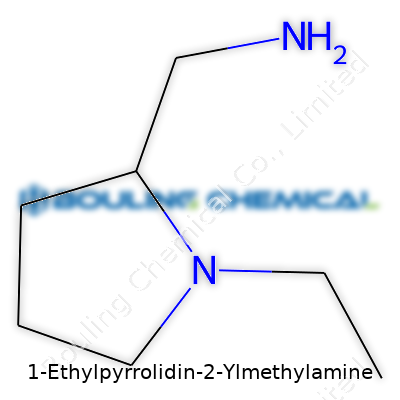
Chemistry names often feel like a puzzle. 1-Ethylpyrrolidin-2-ylmethylamine sounds like something you’d stumble over in a pharmacy textbook, but it breaks down into clear parts. The “pyrrolidine” part signals a five-membered ring containing one nitrogen atom. Picture a tiny hoop, almost like a friendlier, fatter cousin to the classic benzene ring. Toss in an “ethyl” group on the first carbon, and there’s a little tail. Next, tag on a “methylamine” group to the second position—that brings in a small branch containing a nitrogen atom with two hydrogens.
The backbone here is pyrrolidine: a ring with four carbons and one nitrogen, all single bonds, so the ring forms a puckered, squishy shape rather than a flat pancake. Place the ethyl group on carbon one—just a two-carbon stick dangling off that first position on the ring. Then, attach a methylamine group (–CH2NH2) on carbon two, branching away. If I sat down to sketch this, I’d start with a pentagon, check off my carbon numbers moving clockwise, then stick on the ethyl and methylamine groups. There’s no symmetry here, and those side branches are far enough apart to dodge each other.
Getting to know these structures isn’t just for chemists sitting behind lab benches all day. Medicinal chemistry relies on these little tweaks—the difference between carbon one or two can transform how a molecule behaves in the body. In the case of 1-ethylpyrrolidin-2-ylmethylamine, shifting the amine or changing the ring can flip the activity or change how quickly the body breaks it down. Pharmaceutical researchers study molecules like this to see if they fit certain biological receptors—sometimes chasing pain relief, sometimes targeting mental health.
These modifications can also play into safety. Adding an ethyl group or a little amine can mean a big difference between a medicine and a toxin. Drug designers navigate this every day, fine-tuning pieces of a molecule for better effect or fewer side effects. A structure map becomes almost like a street grid, guiding each next step.
Most people won’t ever see a flask of 1-ethylpyrrolidin-2-ylmethylamine. Still, these tiny changes in molecular structure echo out to hospitals, clinics, and pharmacies. Understanding these structures makes it easier to spot counterfeit drugs, develop safer prescriptions, and teach new chemists the creativity behind making medicine.
Technology helps build these molecules faster and check their behavior before candidate drugs get anywhere near a patient. Programs that draw and predict chemical activity combine with safety checks, trying to keep new medicines both effective and safe. But all those big systems start with someone being able to look at a name like 1-ethylpyrrolidin-2-ylmethylamine, break it down, draw it out, and see what makes it unique.
Supporting deeper familiarity with chemical names and structures helps everyone from high school students to industry pros. Training programs and clear graphics can give more people a chance to see the meaning in a name, spot key risks, and understand why one detail in a structure can make all the difference. Research journals and online databases could do a better job providing interactive drawings, not just long lists of letters. And public health outreach can help non-scientists learn to value molecular detail, knowing that every little branch or ring sets the stage for health and safety in daily life.
Nobody likes surprise headaches, burning eyes, or blotchy rashes from a lab session. Even in places with shiny new equipment, I’ve seen co-workers get careless with a bottle they "thought" was safe. One slip, and you end up consulting a material safety sheet for a compound you wish never crossed your desk. I’ve come across 1-Ethylpyrrolidin-2-ylmethylamine in a research setting, and the label alone tells you—this isn't something for kitchen chemistry.
This compound finds its way into organic synthesis and sometimes turns up in pharmaceutical labs as part of new molecule development. Its structure hints at reactivity, with an amine group and pyrrolidine ring. Being a small molecule with an alkyl amine, skin can absorb it, and its vapors hit the eyes faster than some realize. PubChem and relevant chemical catalogs don’t always spell out every possible risk, but they provide key data: moderate toxicity, flammability, risk of causing burns, and poorly documented long-term health effects.
I've read through incident logs where someone, thinking “it’s just another amine,” handled it without eye protection or even gloves. Minutes later, they felt a burning sensation and ended up with irritated skin for days. Serious exposures to amine compounds in general can cause breathing problems, central nervous system effects, and in the longer term, liver or kidney damage. Ventilation and protective barriers always outmatch regret.
Trust and safety in a lab start with how we treat every chemical. Peer-reviewed chemical safety resources, from the American Chemical Society (ACS) to the National Center for Biotechnology Information (NCBI), flag amines—especially ones that aren’t widely studied—for extra caution. Lack of detailed chronic exposure data means guessing is off the table: experienced chemists treat 1-Ethylpyrrolidin-2-ylmethylamine like any other potentially hazardous substance. I keep gloves—nitrile, not cheap latex—within arm’s reach, and always protect my eyes. Fume hoods aren’t decorative; they're essential.
There’s also the bigger picture. Good chemical hygiene stops small accidents from spiraling into legal or health nightmares. Regulations in Europe and North America expect employers to train their people, label chemicals clearly, and maintain emergency equipment. OSHA won’t care if a tech “didn’t think it was dangerous.” Chemical accidents hurt people and shut down research.
Lab managers and educators bear the responsibility for more than their own safety. Younger researchers watch and learn. Skipping a step signals that cutting corners is fine, even when it isn't. Sharing updates from trusted bodies like the ACS and local poison centers goes a long way. I've worked in spaces where safety sheets are within reach, emergency eyewash is clear of clutter, and everyone has practiced spill drills. That doesn’t happen by accident.
In my experience, strong teamwork beats solo risk-taking every time. Announce the hazards when new chemicals arrive. Make sure everyone’s got gloves and goggles. Keep lab areas clean. Confirm the ventilation is running. Dispose of leftovers safely. Teach what the safety sheet leaves out. Only then do people trust each other—and the chemistry they create.
People usually don’t spend time thinking about small, specialized chemicals like 1-Ethylpyrrolidin-2-Ylmethylamine until things go wrong. Most professionals who work with substances like this know these aren’t pantry items. There’s a real need to treat them with care and respect. The smallest slip in handling or storing these compounds can turn an ordinary workday into a disaster.
Many chemicals react to changes around them—heat, light, humidity. This amine doesn’t take kindly to heat. Storing it in a cool place limits the chances of container gas build-up, leaks, or worse, sudden reactions. Direct sunlight shouldn’t hit storage areas, either. I’ve seen windows cause more trouble in chemical storage than anyone likes to admit.Proper airflow makes a difference. Fumes from volatile amines build up fast. Shelving them in a closed cabinet with no fans or vents practically invites trouble. That’s a lesson hammered in by anyone who’s spent time in lab management or chemical manufacturing. Use cabinets with active ventilation or in rooms with built-in extraction fans. That way, nobody has to face rogue fumes at the end of the day.
1-Ethylpyrrolidin-2-Ylmethylamine belongs in tightly sealed, chemical-grade bottles—usually HDPE or glass. Left in containers that aren’t resistant, this amine might corrode, stain, or even eat through the plastic over time. I’ve seen poorly labeled bottles get shuffled or mixed with other chemicals, leading to confusion and real hazards. That’s why labeling must be crystal clear, with both the chemical name and date. Putting the label on the cap doesn’t help if the bottle’s lying down or the ink wipes off.
Mixing the wrong chemicals causes health risks and environmental headaches. Amines react sharply with oxidizers and acids. Storing them side-by-side invites a messy situation. Arranging chemicals by compatibility makes life easier and keeps storage areas safer. I once walked into a store where amines, acids, and peroxides shared a shelf—and it made everyone nervous. Industry guidelines recommend spacing such chemicals far enough apart so they don’t play off each other if something spills or vapor seeps out.
Accidental access by untrained staff, students, or curious visitors becomes less likely with locked, access-controlled spaces. In my experience, open-access shelving in small labs almost always led to problems down the line.Spill kits, eye wash stations, and showers need to stand nearby—no hunting around for them in a pinch. Amine vapors irritate eyes and skin very quickly. Fast, easy response cuts both injury and clean-up time.
Following solid storage practices prevents lost product, injuries, and fines for non-compliance. It’s not just about ticking a regulatory box—neglect risks lives, equipment, and science itself. I’ve worked in labs where ignoring basics led to confusion and wasted money. Good storage habits cut down on accidents and wasted stock, freeing people to focus on their research and results.
Companies and labs storing chemicals like 1-Ethylpyrrolidin-2-Ylmethylamine do well by making these safety habits routine. Regular spot checks, solid training, and keeping the right equipment close transform a storage room from a risk zone into a reliable workspace.
Tracking down a specialty chemical in today’s market isn’t like ordering office supplies. My own research into niche compounds has shown how availability, safety, and legality will make just as much difference as price or shipping speed. 1-Ethylpyrrolidin-2-ylmethylamine falls straight into this category—a compound not stocked at your local store, nor casually shipped around the world. Instead, specialty chemicals demand you know exactly where you stand, and where you may go wrong.
For anyone working in chemistry or pharmaceuticals, sourcing a raw ingredient isn’t new. Many manufacturers publish catalogs with chemicals like this, targeting established customers in science and research. Sigma-Aldrich, Thermo Fisher Scientific, and TCI regularly list hundreds of thousands of compounds, but rarely anything without legitimate industry backing. Why? Medications, lab reagents, and synthetic intermediates easily find misuse when sold without restrictions. If you’ve walked through that procurement process, you’ve dealt with requests for end use, compliance documentation, and licenses.
Demand for 1-Ethylpyrrolidin-2-ylmethylamine comes with baggage: it’s not listed as a household product, nor is it something hobbyists order without clearance. Chemists looking to acquire it usually prove their credentials, provide purchase justifications, and agree to end-use declarations. Most reputable suppliers reject orders lacking credentials. This safeguards against two big risks: untrained buyers suffering chemical exposure and compounds ending up in the wrong applications. These protocols don’t pop up by chance—they mirror growing international agreements designed to restrict chemicals with dual-use risks.
Online searches toss up sketchy “gray market” sites that skip the safeguards. I’ve seen too many cases where these sites offered everything but proof of legitimacy, and those who gambled either lost money or jeopardized their safety. No real verification, no regulatory oversight, and, for the unlucky, customs confiscation or legal investigation. Turning to message boards filled with guidance to “just Google a supplier” only adds to the problem. These shortcuts rarely work for professionals or responsible researchers.
No matter what, buyers should always start by checking local laws before reaching out to suppliers. Many governments publish lists with substances that need extra permits or that simply cannot be imported by individuals. Direct contact with local science distributors or licensed chemical inventory managers helps sort out what’s possible and what’s prohibited. Legitimate suppliers will walk you through the paperwork and help review export controls. These steps might sound tedious, but from my own projects, skipping legal checks often leads to much bigger problems.
It pays to connect with professionals—actual chemists, pharmacists, or licensed importers. These experts know both the regulatory landscape and the best vetted sources. Some academic institutions even maintain their own purchasing relationships to protect both the buyer and the supplier. Asking for guidance here brings experience rather than guesswork. A lab manager’s support opened doors for me that online catalogs never could.
The lesson is clear for anyone who values legal protection and product safety: work with registered suppliers, follow the law, and collaborate with professionals. Sourcing chemicals responsibly isn’t just paperwork—it’s how research, industry, and society avoid needless risk. Anyone pursuing legitimate scientific progress should invest in genuine supply chain relationships, not shortcuts through search engines or anonymous forums.
| Names | |
| Preferred IUPAC name | N-Ethylethane-1,2-diamine |
| Other names |
1-ethyl-2-(aminomethyl)pyrrolidine 1-ethylpyrrolidin-2-ylmethanamine |
| Pronunciation | /ˈwʌn ˈiːθɪl pɪˌrɒlɪˈdiːn tuː ˈɪlˌmɛθɪl əˈmiːn/ |
| Identifiers | |
| CAS Number | 948292-80-2 |
| 3D model (JSmol) | `CCN1CCCC1CN` |
| Beilstein Reference | 172388 |
| ChEBI | CHEBI:189827 |
| ChEMBL | CHEMBL2174389 |
| ChemSpider | 13970308 |
| DrugBank | DB14110 |
| ECHA InfoCard | EC 810-498-1 |
| EC Number | 260-141-8 |
| Gmelin Reference | 13466817 |
| KEGG | C18622 |
| MeSH | D041262 |
| PubChem CID | 10184467 |
| RTECS number | HE1425000 |
| UNII | ZM0W5VF015 |
| UN number | UN3276 |
| CompTox Dashboard (EPA) | DTXSID40708922 |
| Properties | |
| Chemical formula | C7H16N2 |
| Molar mass | 128.215 g/mol |
| Appearance | Colorless liquid |
| Odor | Amine-like |
| Density | 0.957 g/cm3 |
| Solubility in water | soluble |
| log P | -0.02 |
| Vapor pressure | 1.52E-1 mmHg at 25°C |
| Acidity (pKa) | 10.75 |
| Basicity (pKb) | 4.06 |
| Magnetic susceptibility (χ) | -63.34×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.484 |
| Viscosity | 1.06 cP at 20 °C |
| Dipole moment | 3.25 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 274.1 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | N06BX15 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. |
| GHS labelling | GHS02,GHS07 |
| Pictograms | GHS06,GHS08 |
| Signal word | Warning |
| Hazard statements | H302, H312, H332 |
| Precautionary statements | P261, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 82.6 °C |
| Autoignition temperature | 320°C |
| LD50 (median dose) | LD50 (median dose): 1390 mg/kg (rat, oral) |
| NIOSH | LM5425000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 30-100 µg/L |
| Related compounds | |
| Related compounds |
Pyrrolidine N-Ethylpyrrolidine 2-Pyrrolidinemethanamine N-Methylpyrrolidine 1-(2-Aminoethyl)pyrrolidine |