Years ago, piperidine derivatives began attracting attention from researchers and industry alike. Chemists in the mid-20th century looked for ways to fine-tune nitrogen-based heterocycles, and 1-Ethylpiperidine quickly stood out. Early syntheses focused on expanding the range of piperidine analogs beyond what natural sources could provide. Soon, as demands increased in pharmaceuticals and specialty chemicals, the ethnographic spread and study of these six-membered rings allowed 1-Ethylpiperidine to move out of the laboratory and into factories, making it a staple in the toolbox of organic and medicinal chemistry.
1-Ethylpiperidine shows up as a colorless, oily liquid, recognizable by its strong amine scent. Industry relies on it not just for what it is, but for what it can become—its chemistry opens paths to more complex molecules, especially in drug synthesis and fine chemical production. Many suppliers deliver this compound in sealed containers with nitrogen blanketing to keep out moisture and oxidation, extending shelf life and helping users avoid unwanted side reactions.
This compound boils at about 135–137°C and sports a melting point well below room temperature, making it a liquid in most working conditions. Water does not dissolve it easily, but it mixes smoothly with organic solvents like ether and benzene. At the molecular level, the ethyl group attached to the nitrogen atom increases lipophilicity, which influences how it interacts with other chemicals. With a molecular formula of C7H17N and a molecular weight around 115.22 g/mol, 1-Ethylpiperidine packs a punch in a small package, offering a balance of volatility and stability that attracts attention in both research and industry.
Most suppliers label containers with purity levels, usually sitting above 98%, verified by gas chromatography or HPLC. Labels also display the CAS number 766-09-6 and hazard statements based on GHS guidelines. Proper identification helps prevent mix-ups in the lab, especially among students and early-career chemists where handling errors raise safety risks. Demands for traceability also push companies to include lot numbers and production dates, ensuring end-users can track quality back to the original batch.
Producers typically build 1-Ethylpiperidine through alkylation of piperidine using ethyl halides under basic conditions. Engineers favor this direct route for its high yields and cost control. The process calls for careful temperature management and strict exclusion of moisture to cut down on unwanted side-products. Clean workups with distillation and washing remove residual bases, salts, or unreacted starting materials, all steps tested by QC labs to meet downstream requirements for purity and performance. If the feedstocks go off-spec, output quality drops, so suppliers enforce tight controls on their inputs.
1-Ethylpiperidine shows versatility in reactions involving nucleophilic substitution, acylation, and formation of quaternary ammonium salts. The nitrogen’s lone pair attracts electrophiles, enabling formation of a wide range of derivatives used in API manufacture or as intermediates for agrochemicals. Chemists leverage its reactivity to insert aryl or acyl groups, extending its reach into areas as diverse as catalysis and polymer science. Access to substituted piperidines often depends on this core molecule, which acts as a springboard for building up molecular complexity.
Across catalogs and scientific literature, names like N-Ethylpiperidine, 1-Ethylhexahydroazepine, and Ethylpiperidine point back to the same structure. These aliases create confusion unless users double-check CAS numbers and structural drawings. Supply chains often track products by tradenames, so it’s common to see regional labeling differences that have to be aligned before scaling up or starting regulatory paperwork.
Handling 1-Ethylpiperidine safely requires more than gloves and goggles. Inhaling vapors can irritate the respiratory tract, while skin contact leads to redness or chemical burns. Spillage on floors or benches leaves a lingering odor and increases fire risk, especially near ignition sources since the flash point is low. Labs install good ventilation, train staff to work upwind of open containers, and store all amines in flammable cabinets away from acids and oxidizers. Risk assessments guide decisions on PPE and waste disposal since improper handling leads to environmental fines and health claims. MSDS sheets specific to the supplier fill in gaps left by general amine guidelines.
The reach of 1-Ethylpiperidine stretches across pharma, agrochemicals, and specialty material sectors. In my experience working on small-molecule synthesis, its role as a precursor to antihistamines and CNS drugs often pulls it off the shelf. For crop protection chemists, it becomes a scaffold for herbicides or insecticides, thanks to the ease with which piperidine rings can get tailored to disrupt metabolism in target pests. Downstream refiners also slot it into roles as a phase transfer catalyst or a capping agent for advanced polymers, where the blend of volatility and basicity changes polymerization pathways.
R&D teams constantly re-examine 1-Ethylpiperidine for uses that go beyond its current portfolio. Academic groups explore modifications to ring systems to unlock antibacterial activity or improve drug delivery. By looking at analogs with different alkyl chains, researchers chase after compounds with new modes of receptor binding or altered metabolism profiles. In the materials space, work continues to build smart surfaces out of piperidine-based monomers that respond to light or heat, making this compound much more than a footnote in organic chemistry textbooks.
Animal studies point out some hazards linked to overexposure: liver and kidney changes show up at high doses, and acute eye or skin exposure leaves lasting burns. Regulatory agencies track these findings, setting occupational exposure limits so users can gauge safe working ranges. Although full long-term toxicology data for every derivative remains incomplete, human case reports already highlight the need for strong ventilation and prompt spill cleanups. Wastewater guidelines also force industry to treat residues before discharge, keeping amine-loaded streams out of surface water ecosystems. These controls serve as a safety net while research teams look for less hazardous synthesis routes.
Looking ahead, 1-Ethylpiperidine’s story runs parallel to the trends shaping synthetic organic chemistry. As demand for piperidine-based pharmaceuticals and next-generation polymers grows, so does the need for reliable, greener ways to produce and modify this molecule. Teams race to discover biocatalytic or solvent-free routes, cutting emissions and waste. Digital chemistry platforms help model the reactivity and downstream effects of piperidine analogs, trimming years off the drug discovery pipeline. Industry watchers expect to see more applications emerge as researchers discover new biological activities linked to piperidine cores and as end users push for safer, more sustainable sourcing throughout the value chain.
Pharmaceutical labs wind up needing dozens of different building blocks to create potential treatments. 1-Ethylpiperidine shows up on plenty of chemical shelves for this reason. In my years walking labs and scanning through medicinal chemistry projects, I kept noticing how often simple piperidines like this one serve as an early step in medicinal chemistry. Chemists grab this molecule and add extra structures onto it, aiming to adjust drug behavior in the body or improve safety. You find derivatives playing a part in antihistamines, antidepressants, and antipsychotic drugs. I once spoke with a medicinal chemist running a pilot project for new pain relief candidates, and he pointed to ethylpiperidine as the backbone shaping how those new molecules would move through the body.
This compound doesn’t usually get the spotlight for its own sake—it acts as a helper along the chain. In organic chemistry, people turn to 1-ethylpiperidine as a starting point. I remember one synthesis project from my time consulting for a specialty chemicals team—they used it as an intermediate to make new agricultural chemicals aimed at improving pest control without tanking soil health. Synthesis teams prize molecules that help them control reactions without bringing too many unpredictable side products, and this one delivers the stability needed for reliable output.
The six-membered ring makes it handy for building more complicated structures in everything from crop treatments to polymer additives. Looking over patent literature, especially the agricultural patents coming out of Asia and Europe, I saw plenty of processes using ethylpiperidine for these synthetic steps. Factories producing synthesis intermediates in the tens-of-kilograms range rely on it for its balance of volatility and ease of handling.
Think of all the substances that keep machinery humming, from rust inhibitors in pipelines to certain battery solvents. 1-Ethylpiperidine pops up in some of these as a performance additive. Folks working on new batteries test out a lot of nitrogen-containing rings, searching for stability or conductivity without driving up the price. During a visit I made to an R&D center focused on specialty lubricants, their product manager explained that additives like this help keep sensitive equipment running by providing just the right level of breakdown resistance.
While 1-ethylpiperidine brings useful traits, it doesn’t belong anywhere near food or direct consumer products. The Material Safety Data Sheets call for solid gloves and proper ventilation. I’ve sat in on enough safety briefings to know that one slip, especially in scale-up facilities, can lead to a headache for the whole team. This is why most of its buyers are advanced chemical firms or academic research centers with trained staff.
More scrutiny lands on chemical intermediates every year. I see stricter rules from governments seeking to lower worker exposure and environmental waste. Sourcing managers now prefer intermediates that break down safely after use or that chemists can reuse in closed-loop reactions. Several start-ups I’ve watched are experimenting with “greener” syntheses of piperidine derivatives to lower the impact of specialty chemical manufacturing.
Getting more transparency around sourcing and waste disposal helps keep these chemicals doing good rather than harm. Investments in process improvement—automation, waste capture, and better personal protective equipment—can shrink risks, protect workers, and keep the benefits coming for pharma and material science. From my experience, collaboration between industrial chemists, safety experts, and regulators is the real engine moving the field forward.
1-Ethylpiperidine carries the chemical formula C7H15N. Its molecular weight stands at 113.20 g/mol. For anyone who’s spent time in a chemistry lab or read ingredient labels, seeing a string of letters and numbers like this isn’t new. But for a lot of people, these markers seem more like passwords than identifiers. In truth, they tell us a lot about the substance and its character.
1-Ethylpiperidine does more than give chemists one more thing to memorize. It plays a role in the world of organic synthesis. Think of it like that reliable wrench in a toolbox — not flashy, but essential when building bigger, more useful molecules. Pharmaceutical researchers tap compounds like 1-ethylpiperidine to build new drugs. When you realize how complicated drug development can be, you come to appreciate even the simplest building blocks.
This chemical’s structure—built from a six-carbon piperidine ring with an ethyl group kicked onto the first carbon—gives rise to its reactivity. Its nitrogen atom also draws interest. Nitrogen atoms often act as landing spots for other molecules, helping researchers link one piece to another in a controlled way. The piperidine family, including this compound, pops up again and again in drugs targeting everything from mental health to pain management.
I remember the first time I worked through molecular weights in the lab. Misjudging those numbers meant my calculations fell apart, my synthetic yields dipped, and I ended up with more questions than answers. Getting a handle on both the formula and the weight of a compound isn’t about showing off on a quiz; it determines if you have what you need to plan a reaction, measure out amounts, and, most importantly, check if your experiment adds up.
1-Ethylpiperidine serves as a good reminder here. With a formula like C7H15N, you know it packs seven carbons, fifteen hydrogens, and a single nitrogen. No more, no less. That gives you a backbone for figuring out how it can interact with other chemicals and what kind of molecules you can build on top of it. Look up its molecular weight, and you know how much to weigh out. Any error at this stage ripples through the rest of your work.
Technical details alone don’t keep anyone safe. I’ve worked with nitrogen-containing molecules, and even simple ones can have unexpected effects. Labs run on strict protocols for a reason. Proper labeling, thoughtful storage, and good ventilation keep risks low. It doesn’t take long for bad habits to creep into routine, so keeping up with safety guidelines builds a culture of trust and attention to detail. The chemistry community always pushes for better training, updated safety sheets, and clearer communication about hazards, even with common molecules.
The more I see drug development and material science evolve, the more I realize the need for education and broad access to clear data. Reliable resources allow scientists to jump into research without guesswork holding them back. Efforts to standardize chemical data — clear formulas, accurate molecular weights, robust hazard communication — help drive discovery. By keeping information accessible and understandable, everyone from students to seasoned researchers can work smarter, not harder.
References:A lot of folks working with 1-Ethylpiperidine know it's handy in labs, especially with organic synthesis and specialty compounds. It fits right into the world of pharmaceuticals and fine chemicals, but the risks stick close, too. Exposure—even a small amount—can lead to skin and eye irritation and can mess with your lungs if you breathe in the vapor. I’ve spent years in labs where one mistake can mean serious downtime, so respecting the basics isn’t just smart; it keeps you and your coworkers safe.
Every time I’ve handled volatile amines, I double-check my personal protective equipment. For 1-Ethylpiperidine, gloves made from nitrile rubber have held up well, and splash goggles keep you covered. Lab coats, closed shoes, and chemical-resistant aprons help prevent splashes from reaching your skin. In small, stuffy labs, the fumes have a sneaky way of lingering, so I use proper respiratory protection if there's any doubt about air quality. Even a tiny spill can stink up the whole place and irritate your throat.
Open up a bottle in a closed room and you'll know fast—1-Ethylpiperidine's fumes aren’t friendly. Fume hoods or local exhaust ventilation do the heavy lifting. I found that simple tricks like keeping the vial near the back of the hood really cut down on the chance of breathing in vapors. More than once, a quick whiff let me know I didn’t have the air moving quite enough, so checking those airflow monitors at the start of each day always pays off.
Flammable liquids like this have a way of causing trouble if they’re stashed thoughtlessly. Solid shelving helps, but I also make sure chemical containers stay sealed tight when not in use. The material likes to react with strong oxidizers and acids, so I keep those stored in separate, clearly labeled cabinets. From experience, even slight leaks in a bottle can lead to awful odors and corroded shelves if left unchecked.
Every spill protocol I’ve seen stresses speed and thoroughness. I keep absorbent pads nearby and neutralize minor spills with inert materials like vermiculite. After cleaning up, you want to ventilate the area well—fumes hang around far longer than you'd expect. Any exposure to eyes or skin demands a steady, gentle stream of water for at least fifteen minutes, with a trip to the clinic if anything feels wrong afterward. I’ve seen coworkers brush off splashes, only to regret it later.
Pouring leftover chemical down the drain risks water contamination and puts stress on municipal treatment plants. I store all waste in safe, labeled containers and arrange collection with licensed hazardous waste firms. Local regulations guide disposal practices, so it pays to check those details before moving or discarding unused supplies.
Anyone who thinks gloves and goggles slow things down hasn't dealt with the fallout from an accident. Taking a few extra minutes before unpacking a bottle or decanting into a reaction flask saves hours of cleanup—and protects your health for the long haul. With chemicals like 1-Ethylpiperidine, small mistakes can snowball, so being methodical is less about rules and more about respecting what’s in the bottle.
1-Ethylpiperidine has made a mark as a versatile organic compound in chemical manufacturing and research. This substance brings a mix of volatility and reactivity that calls for genuine care toward safety. Having handled similar nitrogen-based chemicals before, I know spills can go from minor events to major hazards without proper respect for storage and logistics.
A big lesson from my own lab days: never underestimate the risks posed by flammable liquids. 1-Ethylpiperidine fits into this category. It catches fire at a low temperature, releases vapors easily, and can irritate skin, eyes, and airways. Keeping this compound in a cool, dry, well-ventilated spot away from sunlight helps prevent most accidents. Metal storage cabinets with proper grounding give extra security, since static discharge can ignite flammable vapors.
Glass bottles with sealed lids keep air and moisture out, avoiding unwanted chemical changes. Labels and hazard warnings must stay clear and permanent—after a long day, nobody wants to play guessing games with dangerous substances. Regular checks for leaks or damage reduce chance of an unwanted surprise.
Especially in warmer climates, vapor buildup inside storage rooms poses a real threat. Exhaust fans and spark-proof ventilation systems keep air moving and reduce the odds of inhalation or flammable concentrations. My own experience with ammonia-based solvents convinced me that stale air mixes poorly with volatile chemicals.
In fire emergencies, sprinklers designed for chemical storage help slow the spread, but nothing beats planning ahead. Dry sand or foam extinguishers offer a better response than water for fires involving this compound.
Transportation brings a different set of challenges. Packagers usually favor strong, tight containers—think metal drums with tight gaskets or heavy-duty polyethylene bottles. Double-sealed bags or shrink-wrap can guard against sudden spills. Trucks should lock these containers upright, with clear hazard labels on every piece.
Drivers moving these chemicals hold a big responsibility. Proper documentation—including the material safety data sheet—travels with each shipment. During transport, any temperature-controlled section of a truck or container keeps the substance stable. It’s not complicated, but cutting corners leads to risk.
No storage or shipping solution beats well-trained staff. I’ve watched new colleagues become diligent about glove use, eye protection, and avoiding sparks after just one safety briefing. Spill kits should sit near every storage site and on any vehicle moving this type of load. Training covers not just cleanup, but how to call for help and keep others out of harm’s way.
Planning includes backup: fire alarms, eye wash stations, and emergency showers, because accidents rarely announce themselves. Chemical exposure isn’t forgiving, so preparation keeps people safe.
Safer packaging materials lower the impact if a container cracks or leaks. Digital monitoring now alerts staff to temperature swings or vapor leaks, letting problems get fixed before they worsen. Investing in these improvements isn’t about overkill—it pays off when safety margins get tight.
Strict guidelines, vigilant staff, and solid physical measures keep 1-ethylpiperidine from causing trouble. Respect for the substance and practical habits, not just fancy technology, still do most of the work.
Anybody searching for 1-Ethylpiperidine in drums or barrels likely works with chemical synthesis, pharmaceuticals, or agrochemicals. It’s a compound used for a reason—this piperidine derivative makes its way into catalysts, drug intermediates, and specialty chemicals. If bulk access comes up, it’s because a project or process truly needs scale; weighing grams just doesn’t cut it for manufacturing or development. In my experience, chemicals like this rarely get bought in a vacuum. Procurement teams ask one another: how much can you get, what will it take, and, maybe most urgently, how do you keep it safe?
Major suppliers and distributors do offer 1-Ethylpiperidine in larger quantities. Look at the catalogs of known chemical suppliers—Alfa Aesar, Sigma-Aldrich, TCI, and their peers. Contacting them directly, buyers often get prices for as little as a few hundred grams or as much as 50 kilograms (sometimes more). Industrial buyers, especially those outside North America and Europe, might find local traders or OEM factories able to arrange deals in the hundreds of kilograms. What counts most: trust and documentation. Reliable companies supply a certificate of analysis, batch traceability, and up-to-date safety data. Shipments must satisfy the legal standards of customs, hazardous materials transport, and workplace safety. This adds real peace of mind for everybody downstream.
Packaging matters for chemicals like 1-Ethylpiperidine. It isn’t just a box or a drum. This is a liquid with legitimate flammability concerns and a tendency to give off a strong odor. In my own work, nothing throws off a lab or storage area like a leak. Typically, large orders ship in high-density polyethylene (HDPE) drums or bottles, while lighter steel drums sometimes show up for ultra-demanding conditions. Volumes can range from one-liter bottles up to 200-liter barrels. For middle ground, five- or 20-liter carboys prove handy. Smaller samples often ship in glass—though that’s really only practical for evaluation or R&D, not for continuous plant use. Deliveries include inner liners and secondary containment if regulations ask for it. Seams and closures are checked to avoid spills in shipping (nobody likes returns or regulatory headaches).
Bulk sourcing cuts costs per unit and reduces the hassle of ordering again and again. Safe, practical packaging stops waste, product loss, and risk of regulatory fines. If something like 1-Ethylpiperidine contaminates a warehouse, the cleanup can outstrip the cost of the chemical itself. Sound buying habits—get the specification, confirm compatible packaging, and verify UN credentials for hazardous liquids—keep people and budgets safe. Choosing a familiar supplier based on reviews and industry reputation goes a long way. Standardized drum sizing lets manufacturers automate pumping, batching, and storage too, saving money and time. Anyone who’s seen a facility deal with a mystery drum or the wrong container knows the value of nailing this step up front.
One way the industry can improve: simplify traceability and online documentation. QR codes on packaging could link directly to batch reports and real-time transport updates. More coordination between vendors and buyers can fine-tune container sizes to reduce partial waste on delivery. Open channels between suppliers and end users foster safer use and clear expectations. Easy access, solid packaging, and reliable paperwork give operations teams fewer headaches and let them focus on what actually matters: production, not paperwork or spills.
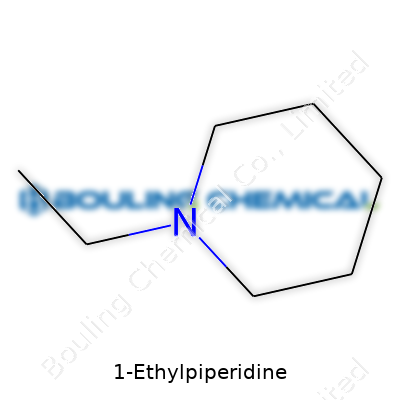
| Names | |
| Preferred IUPAC name | N-ethylpiperidine |
| Other names |
NSC 8776 1-Ethyl-piperidine N-Ethylpiperidine |
| Pronunciation | /wʌn-ˈiːθɪl-pɪˈpɛrɪdiːn/ |
| Identifiers | |
| CAS Number | 5316-69-2 |
| Beilstein Reference | 1041571 |
| ChEBI | CHEBI:18727 |
| ChEMBL | CHEMBL152191 |
| ChemSpider | 17501 |
| DrugBank | DB03163 |
| ECHA InfoCard | 100_008_042 |
| EC Number | 202-930-3 |
| Gmelin Reference | 76149 |
| KEGG | C11759 |
| MeSH | D010993 |
| PubChem CID | 12361 |
| RTECS number | EK8775000 |
| UNII | R3B449221C |
| UN number | UN2401 |
| Properties | |
| Chemical formula | C7H17N |
| Molar mass | 129.24 g/mol |
| Appearance | Colorless liquid |
| Odor | amine-like |
| Density | 0.816 g/mL |
| Solubility in water | Slightly soluble |
| log P | 1.82 |
| Vapor pressure | 1.8 kPa (at 20 °C) |
| Acidity (pKa) | 11.2 |
| Basicity (pKb) | 3.05 |
| Magnetic susceptibility (χ) | -54.0e-6 cm³/mol |
| Refractive index (nD) | 1.422 |
| Viscosity | 0.778 cP (20°C) |
| Dipole moment | 1.35 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 309.4 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -45.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3933.7 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | HCCH2N1CCCCC1 |
| Signal word | Warning |
| Hazard statements | H226, H302, H314 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P280, P303+P361+P353, P370+P378 |
| NFPA 704 (fire diamond) | 2-3-1 |
| Flash point | 38 °C |
| Autoignition temperature | 255 °C |
| Explosive limits | 1.3-7.2% |
| Lethal dose or concentration | LD50 (oral, rat): 306 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 310 mg/kg |
| NIOSH | SKC05740 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 200 kg |
| IDLH (Immediate danger) | IDLH: 200 ppm |
| Related compounds | |
| Related compounds |
1-Methylpiperidine 1-Propylpiperidine 1-Butylpiperidine Piperidine 2-Ethylpiperidine |