Chemists started paying closer attention to piperidine-based compounds in the early 20th century, piecing together their structures from the roots of alkaloid research. 1-Ethylpiperidin-3-ol caught more eyes as labs searched for new building blocks in pharmaceuticals and agrochemicals. Its relationship to the backbone of piperidine, familiar in morphine research and synthetic routes, positioned it as a chemical of interest. Laboratories looking to tune biological activity realized that swapping out a hydrogen for an ethyl group on the nitrogen, and adding a hydroxy group at carbon-3, made for a versatile skeleton that could cross boundaries between drug synthesis, organic chemistry, and even materials science. Over time, synthetic routes became more reliable, and 1-ethylpiperidin-3-ol moved from rare specialty product to a staple chemical for research and small-scale manufacturing by the late 1990s.
1-Ethylpiperidin-3-ol brings a unique ring structure to the table, combining heterocyclic stability with sites ripe for functionalization. Through years of work, chemists found it easy to introduce various modifications on both the nitrogen and the hydroxyl, which opened doors to making a wide range of derivatives. The molecule usually appears as a clear to pale yellow liquid, sometimes forming as a crystalline solid at lower temperatures. Research-grade versions get delivered tightly sealed, the smell faintly amine-like, and shelf life stretches to several years if stored out of direct sunlight. Its dual functionality lets researchers plug in new chemical groups or link with more complex molecules. This quality keeps it popular across labs in medicinal chemistry, synthetic intermediates, and, in some countries, as an additive in advanced polymers.
True to its class, 1-ethylpiperidin-3-ol shows high miscibility with ethanol, ether, and most polar solvents, but stands stubborn against dissolution in heavy hydrocarbons. It melts around 56–60°C, with a boiling point climbing just above 200°C under standard conditions. The molecule keeps pH stability in the range of 5 to 8. Its amine group, secondary in nature, picks up mild protonation in acidic environments, while the alcohol group introduces hydrogen bonding that impacts both solubility and reactivity. Under regular lab conditions, it hardly reacts with atmospheric oxygen or moisture, making it a low-hassle compound to store and handle. Under UV light, the compound holds up, and no noticeable decomposition happens until temperatures march above 120°C.
Suppliers list 1-ethylpiperidin-3-ol under its CAS number 54999-38-9 and often add EINECS and PubChem identifiers for cross-referencing. Product labels cover batch purity, common levels close to or above 98% for research purposes. Residual solvents (often ethanol or dichloromethane) show up as trace entries, since synthesis stages may leave milligram amounts. Containers carry stability warnings for light and temperature, and chemical safety codes match GHS standards, flagging issues related to skin and eye contact. Some producers stamp QR codes linking directly to digital SDSs for real-time safety information and traceability, reflecting a shift in how chemical handlers meet audit requirements for quality and safety.
Most bulk 1-ethylpiperidin-3-ol comes out of reductive alkylation or a Mannich-type reaction, with ethyl iodide or bromide serving as the ethyl source for N-alkylation, and protected intermediates employed to avoid over-alkylation. Steps proceed under nitrogen to keep oxidation at bay, and hydrogenation catalysts speed up the process. Once the ring structure forms, selective reduction provides the hydroxyl at the third position. Crystallization using hexane-ethyl acetate helps isolate the product, with filtration and rotary evaporation yielding the final compound. Some academic labs trial micro-flow setups to push returns higher and control reaction temperature more tightly, cutting down on production time and material waste.
The molecule stands ready for a variety of downstream reactions. Acylation of the hydroxyl group gives protected esters useful in multistep pathways. N-alkylation and N-acylation shift its pharmacological activity and tune solubility, attracting research in CNS-active agents and intermediate drug scaffolds. Oxidation at the hydroxyl generates ketone analogs, which act as stepping stones for other heterocycles. The ring's nitrogen can anchor larger groups, providing jump-off points for macrocycle synthesis. Halogenation at positions 2 or 5 on the ring helps researchers tweak electronic character and biological binding profiles. Each reaction highlights a different side of 1-ethylpiperidin-3-ol’s utility, echoing why researchers return to it throughout synthetic projects.
Chemists might bump into this compound under names like N-ethyl-3-hydroxypiperidine, 1-ethyl-3-piperidinol, or its registry label 54999-38-9. Depending on country or supplier, labels may shorten to EtPip-3-ol, EHP, or simply "ethylpiperidinol" in informal settings. None of these alternate titles changes the underlying chemistry or application, but variations in nomenclature can challenge sourcing for those new to heterocycles. Professional catalogs keep cross-references handy to cut down on confusion.
Direct contact with 1-ethylpiperidin-3-ol can trigger mild skin and eye irritation, an experience echoed in standard MSDS documentation. I found lab coats and nitrile gloves enough to avoid mishaps, and the liquid vapors rarely rise to concentration levels that worry industrial hygienists. Labs must keep it vented in well-aired hoods, but reports of severe acute toxicity remain rare. For disposal, procedures focus on dilution and incineration, following local rules for organics. Auditors check labels, traceability, and handling logs to ensure compliance with GHS and OSHA. Fire risk stays low thanks to a moderate flash point and high boiling range. Spills mop up with standard absorbents, and I never saw it trigger a lab shutdown for toxicity or reactivity. Accidents tend to result from poor technique rather than inherent danger.
Pharmaceutical development leads the charge, where 1-ethylpiperidin-3-ol bridges precursor chemistry in antipsychotics, anti-infectives, and early-stage painkillers. Researchers in my network also dig into its promise as a chiral auxiliary or catalyst base for asymmetric synthesis, since small tweaks to the ring structure can tip reaction selectivity. Some agrochemical projects slot it in as a backbone for insecticides and herbicides, riding on its ring stability and reactivity. Materials scientists experiment with its inclusion in specialty polymerization, pursuing mechanical or thermal modifications by nesting its molecular ring inside resins. Its use became so versatile I often saw the same chemical lot check in at drug synthesis, crop science, and plastics prototyping over the span of a year.
R&D outfits invest hours mapping the reactivity of both the N-ethyl and 3-OH groups, since both sites stand as obvious targets for linker attachment or chemical tagging. Projects track the molecule's action as a solubilizer and reaction partner in crowded molecular environments. Early computer models struggled to metric its interaction energies, but more recent computational methods lay out predictive routes that save time and material. Research teams in drug discovery notice 1-ethylpiperidin-3-ol ranks high as a privileged scaffold for follow-on molecules. At roundtables, people swap notes on how subtle changes in reaction conditions prompt dramatic shifts in downstream activity. Consistency in supply and reliable analytical data let labs plan longer-term studies, pushing the field ahead in both academia and industry.
Toxicology trials focus on acute oral and dermal exposure, with LD50 values ticking upwards of 500 mg/kg for rats in some reports, although long-term data remains sparse. Cell culture studies hint at mild membrane disruption at higher concentrations but rarely cause runaway cytotoxic effects. Regulatory agencies call for organ-specific screening before considering use in consumer-facing products. In my experience, research teams take a cautious approach by quarantining any waste streams and logging exposure. Animal studies confirm rapid renal clearance and limited bioaccumulation, diminishing environmental hazard concerns. Community demand for environmental safety keeps pressure on improved analytic tracing and transparent reporting.
New directions in medicinal chemistry keep expanding 1-ethylpiperidin-3-ol’s role. With more machine learning tools arriving in synthetic chemistry, researchers predict and trial new analogs faster, looking for compounds with sharper therapeutic profiles and reduced toxicity. The hunger for renewable, low-impact feedstocks spotlights bio-based synthetic routes, and pilot plants test fermentation-based supplies to reduce the environmental footprint of traditional organic synthesis. Platforms developing targeted drug delivery often look to substitute or tag this molecule as a reactive core, opening more routes for functionalized nanoparticles. Advances in microreactor technology hint at continuous-flow production, scaling output without boosting energy use or waste. The next chapter will likely blend classic piperidine know-how with digital design and greener practice, opening the floor to both established labs and newcomers driven by sustainability and precision.
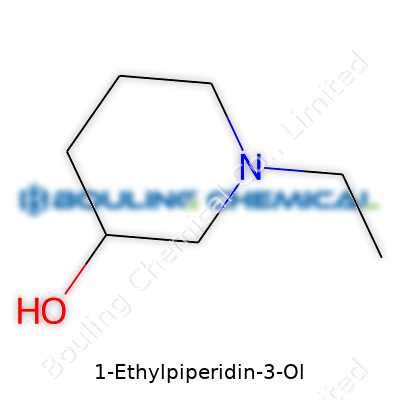
You don’t have to be a chemist to notice that 1-Ethylpiperidin-3-ol isn’t just another random compound. The name itself reveals the backbone: a piperidine ring, sporting both an ethyl group and a hydroxyl group. In everyday terms, piperidine means a six-membered ring made from five carbons and one nitrogen atom. This nitrogen sits at position one. At this spot, an ethyl group (two carbon atoms in a chain) attaches itself. Down at position three, the molecule carries a hydroxyl group, familiar to most as -OH.
Structurally, if you plotted this out, you’d see the ring, nitrogen taking the first seat, the ethyl group jutting out from the nitrogen, and then the -OH clinging firmly to the third carbon of the ring. These changes—adding an ethyl to nitrogen and putting an –OH on the ring—transform the base compound, piperidine, into something with unique properties.
Small tweaks in a chemical scaffold set the scene for new possibilities. With its particular structure, 1-Ethylpiperidin-3-ol brings new physical and chemical features compared to basic piperidine. A hydroxyl group tends to increase water solubility and changes how the molecule interacts inside a biological system. That extra bit helps open the door for applications in making certain pharmaceuticals and fine chemicals.
The pharmaceutical world counts on piperidine derivatives in several common drugs—antidepressants, antipsychotics, and even some painkillers. Their biological activity depends on what sticks off the ring. Adding an ethyl and a hydroxyl creates sites for new chemical connections. Drug designers get excited by this. There’s room to attach more genes for targeting purposes or to improve how a compound travels inside the body. The structure allows researchers to customize a drug’s profile—to improve absorption, limit breakdown in the body, or even cut out side effects.
An extra functional group, like the hydroxyl, means chemists need to think hard about safety and environmental impacts. A compound such as 1-Ethylpiperidin-3-ol may show increased reactivity. It could bond with biological molecules more easily. This property sometimes leads to higher potency in certain therapies, but it also means toxicologists must keep a close eye.
The compound’s structure shapes its journey through manufacturing and waste streams. Hydroxyl groups often make a molecule more biodegradable—but that isn’t always a free pass to declare it safe. I’ve seen how compounds assumed to be friendly because of their structure end up causing headaches, especially after wide environmental release. Chemical vigilance—regular checks, risk assessments, and strong regulation—remains key. The knowledge about this molecule’s structure lets scientists and policy experts plan proper safety plans and disposal methods.
Moving beyond curiosity, scientists can put 1-Ethylpiperidin-3-ol’s structure to work by screening it for new therapeutic uses. At the same time, any potential applications must ride side-by-side with rigorous lab work on environmental impact. Open databases on new compounds’ toxicity and a transparent exchange of results help build trust in how these chemicals are managed.
Getting the structure right gives a head start on every new application or regulation. I’ve noticed that teams who pay careful attention to seemingly small tweaks—like adding an ethyl or a hydroxyl group—tend to avoid big problems down the line. Close study of structure, together with input from chemists and regulators, offers the clearest path to safe innovation.
1-Ethylpiperidin-3-ol has carved out a spot as a building block in drug development. Chemists work with it because its structure lends itself well to producing active pharmaceutical ingredients (APIs). Its presence supplies the right geometry for molecules that treat central nervous system disorders, especially in the discovery phase. Over the last decade, research has shifted toward heterocycles like piperidines due to their strong track record in making safer, more targeted drugs.
Medicinal chemists keep reaching for this molecule to improve molecular interactions with receptors or enzymes. The hydroxyl group and ethyl side chain each change how the molecule fits inside a biological target, influencing potency or side effect profiles. In practice, these fine tweaks make or break early leads as projects move toward animal studies and clinical trials. Data from research labs show that small ring changes on piperidines can impact absorption or blood-brain barrier crossing, both critical for effective medicines.
1-Ethylpiperidin-3-ol has a niche in fine chemical synthesis outside pharma. People use it to add diversity in agrochemical pipelines—things like herbicides, fungicides, or pesticides. Agricultural chemists sometimes need intermediates with precise shape to design active compounds that act only on certain weeds or fungi, reducing harm to beneficial organisms.
In my early years working in chemical R&D, a team used related piperidinols for custom synthesis projects, helping industries pivot quickly if safety or effectiveness issues appeared in testing. Once, a company pivoted their pipeline for a new herbicide after seeing how a molecule with a similar backbone performed in the field. That kind of flexibility can save research budgets and cut down the time to market for urgently needed solutions in farming.
High-performance material companies take advantage of 1-ethylpiperidin-3-ol’s structure. It’s used as a piece in crafting functional polymers and specialty coatings. The nitrogen and alcohol groups serve as points to click onto, helping material scientists design resins with improved flexibility or chemical resistance. This trick has played out in lab-scale batches where temperature or solvent range matters, such as electronics coatings or adhesives.
A year ago, I saw a university group build a prototype for an anti-corrosive coating using a series of piperidinol derivatives. Their results pointed to better rust prevention in humid environments. These gains look small at first but make a difference in infrastructure costs over decades.
The biggest challenge with 1-ethylpiperidin-3-ol comes from supply and safe handling. Skilled chemists deal with volatile organic amines daily, but labs need reliable logistics and storage infrastructure to keep work safe and efficient. Sourcing high-purity material in bulk also presents a puzzle for smaller labs or startups, since price swings can hit cost arithmetic hard.
Green chemistry is something more people want to see. Manufacturing with minimal waste and safer solvents would open more doors, especially for sustainable processes. Investment in safer, scalable manufacturing routes—continuous processing or renewable feedstocks—can make a real difference. Collaboration between public labs and chemical producers could push these improvements along, lowering environmental footprints while keeping industrial progress on track.
Every chemical carries a unique identifying number, known as the CAS (Chemical Abstracts Service) number. For 1-Ethylpiperidin-3-ol, this number is 72514-18-8. It’s not just a string of digits – it plays a big role in research, regulations, and safety. Without these clean identifiers, scientists and manufacturers could confuse substances with similar names. I remember my first chemistry internship; nothing brought more clarity to inventory or literature searches than a dependable CAS number. Mixing up chemicals in the lab isn’t an option when safety’s on the line.
Most people outside of science rarely mull over these numbers. Those of us who work around chemicals can’t avoid them. The right CAS number reveals the legal status, known hazards, and approved uses for a specific compound worldwide. Some governments and regulatory agencies, like the EPA or the European Chemicals Agency, base entire chemical safety protocols on this single number.
Searching for 72514-18-8 leads researchers directly to details about 1-Ethylpiperidin-3-ol, side-stepping confusion from translations or misspelled names. In my own experience managing a chemical stockroom, missing or inaccurate CAS numbers turned quick tasks into headaches. Accidentally ordering the wrong item or storing the wrong bottle in the wrong place invites disaster. A quick scan for this number sorts out those stories before they start.
Creativity in chemistry isn’t just about inventing new compounds; it’s about precision. Registries use the CAS system to prevent duplicate entries, so labs don’t waste resources on already-known substances. Authenticity matters for patents and intellectual property. I’ve seen PhD students rely on this system to prove they’ve developed something novel.
Misinformation can clog up research pipelines. Countless hours disappear when a compound’s identity is unclear – especially in pharmaceutical or biotech research. The CAS number acts like a passport, validating the true nature of a molecule so teams can move onto real work without second-guessing data.
Education stands front and center. Early introduction to the CAS registry and proper labeling can cut down on miscommunication that causes real-world harm. Digitizing chemical stocks helps as well, pairing barcodes and CAS numbers to push toward error-free processes.
Modern research facilities track chemicals on cloud databases tied to CAS numbers, making real-time inventory, hazard tracking, and disposal easier. This system removes guesswork, especially in global companies where one slip creates cross-border confusion. At conferences or when networking, trading just a CAS number opens direct access to verified data—no ambiguity, no language barriers, just shared standards.
Behind every CAS number sits practical reality. For 1-Ethylpiperidin-3-ol and thousands of other compounds, using 72514-18-8 ensures accuracy from the supply chain to safe handling and scientific discovery. Those numbers might look simple, but they bring calm to the sometimes chaotic world of chemicals.
Folks often assume that if something isn’t a household name, it couldn’t do much harm. That’s not the case with many industrial or research chemicals, and 1-Ethylpiperidin-3-Ol stands as a good example. This substance might show up in pharmaceutical labs, among custom synthesis shops, and even when making fine chemicals for specialized uses. I’ve seen plenty of people in science jobs take a closer look at data sheets only after a nasty splash has already happened, so it pays to get ahead of the curve.
Safety data on 1-Ethylpiperidin-3-Ol isn’t always easy to find. There’s limited peer-reviewed information, but what’s out there raises some eyebrows. The chemical belongs to the piperidine family, a group that chemists know can produce skin, eye, and respiratory irritation. Many piperidine derivatives—especially those with small alkyl groups like ethyl or methyl—carry risks including organ damage after long exposures and severe irritation on contact.
Regulatory agencies like OSHA and NIOSH haven’t published specific limits for 1-Ethylpiperidin-3-Ol. That doesn’t give anyone a green light to assume safety. Many of us working in labs and industry know to use the precautionary principle: treat it as hazardous until proven otherwise. The Material Safety Data Sheet (MSDS) points out the risks of inhalation, ingestion, and skin exposure. You only need to look at incidents with similar molecules—such as 1-methylpiperidin-3-ol or 4-ethylpiperidine—to see hospital visits and chemical burns.
Labs and manufacturers rely on standardized safety gear for a reason. I remember working with amine-based solvents in a research setting. Protective gloves, goggles, and a fume hood weren’t optional. Without them, just a few drops could sting your nose or burn your hand. Companies that cut corners on training or safety equipment set up employees for emergency room trips. Even at low concentrations, the volatility of these chemicals allows for easy vapor exposure, which hits the lungs and eyes fast.
The National Institute for Occupational Safety and Health highlights the dangers of similar nitrogen-containing compounds. Chronic exposure raises risks beyond irritation—think liver or kidney problems. I have spoken with researchers who ignored the MSDS and ended up with chronic dermatitis or had to work through headaches for months.
Simple steps offer solid protection. Always wear nitrile or neoprene gloves, since latex often lets small molecule amines slip past. Goggles block splashes, and lab coats add a layer for the unexpected. Never pipette or transfer this chemical on an open bench; a certified chemical fume hood keeps vapors out of the breathing zone. If spills do happen, have an absorbent and neutralizing kit nearby. Emergency eyewash and showers shouldn’t gather dust, either—they really do save vision and skin if things go wrong.
For managers and supervisors, regular training on chemical hazards makes a major difference. Reading Safety Data Sheets may sound boring, but every team member should know where to find them and what’s inside. In daily practice, treating new or obscure molecules like 1-Ethylpiperidin-3-Ol with respect saves businesses from lawsuits and workers from lasting injuries.
People working with chemicals owe it to themselves and their peers to approach every bottle with a healthy dose of caution. Sometimes skepticism isn’t just smart; it keeps us all safe and healthy for the long haul.
1-Ethylpiperidin-3-ol doesn’t show up in most people’s lives, but in chemical labs or production spaces, it gets handled daily. This compound, used in the pharmaceutical and specialty chemical sectors, shows a sensitivity to several environmental factors. My years in lab management have taught me that you can’t beat careful storage—too many accidents start with overlooked containers or improper labelling.
A dry, room-temperature shelf often works for many organics, but this alcohol comes with a moderate volatility, and the chemical can pick up moisture from the air. So, sealed glass bottles go a long way. Polyethylene or polypropylene containers serve as alternatives, but glass never reacts in unexpected ways with solvents. Make sure containers are tightly stoppered—opening up the workspace to a faint whiff of something that shouldn’t escape isn’t just wasteful, it’s a risk.
1-Ethylpiperidin-3-ol isn’t a household name. Yet, just like any low-melting, hygroscopic compound, it tends to signal its presence if left untended. I’ve seen labels fade, and plastic warp from sunlight. UV light exposure encourages breakdown and contamination, so keep the shelving away from direct sunlight. Think of every storeroom as a possible point of failure—shelve this chemical away from acids, oxidizers, and strong reducing agents. A forgotten bottle next to something reactive can invite a mess or, worse, injury.
Classification matters here. This chemical often falls under “irritant” based on skin and respiratory reactions. Inhaling vapors or handling with uncovered skin means possible irritation—more than once, we’ve had to post extra reminders after someone underestimated a “mild” hazard. Don’t store near high-traffic doors, food storage, or shared utility closets.
Scooping out a sample or decanting into another vessel isn’t tricky, yet mishaps often happen during rushed morning setups. My labs established a rule that personal protective equipment—gloves, goggles, full sleeves—always stays on. Clean workspace before and after handling the chemical; residue left behind builds up over time and causes unplanned chemical reactions. Simple practices, such as keeping spill kits nearby, cut down the fear of “what happens if.”
Ventilation means more than a whirring exhaust fan. Use fume hoods whenever handling this liquid for any extended period. Flammable vapors aren’t dramatic in a well-managed space, but venting outdoors through proper filters ensures a workspace stays below occupational exposure guidelines. I’ve checked the OSHA tables—fresh air always wins.
Labs lose chemicals every year to spills, poor seals, and poor record-keeping. Barcoding inventory, doing monthly spot checks, and cleaning the shelving have improved things where I work. Staff training makes a bigger impact than most realize. Staff who lost a chemical due to a leaky cap rarely repeat the mistake, especially after cleanup responsibilities.
In practice, keeping a simple laminated safety sheet on the storage shelf can inform temp workers and seasoned staff alike. Fire extinguishers and eye-wash stations take up little space but make all the difference during emergencies.
At the end of the day, no shortcut ever beats smart, consistent routines. Proper training, regular checks, and plain common sense—these steps keep both people and product safe. That’s the heart of safe chemical storage.
| Names | |
| Preferred IUPAC name | 1-ethylpiperidin-3-ol |
| Other names |
1-Ethyl-3-piperidinol 1-Ethyl-3-hydroxypiperidine 1-ethylpiperidin-3-ol |
| Pronunciation | /ˈwʌn ˈiːθɪl pɪˈpɛrɪdɪn θri ɒl/ |
| Identifiers | |
| CAS Number | 725247-18-7 |
| 3D model (JSmol) | `3D AtomStick model|CCN1CCC(CO)CC1` |
| Beilstein Reference | Beilstein Reference: 0110731 |
| ChEBI | CHEBI:189430 |
| ChEMBL | CHEMBL4151189 |
| ChemSpider | 12396299 |
| DrugBank | DB08398 |
| ECHA InfoCard | 03bb801b-95e8-4b20-96bd-cf64badf7f8e |
| EC Number | 81720-04-1 |
| Gmelin Reference | 126284 |
| KEGG | C19168 |
| MeSH | D010973 |
| PubChem CID | 12571120 |
| RTECS number | UJ8575000 |
| UNII | ED8V1J1PWJ |
| UN number | UN1993 |
| Properties | |
| Chemical formula | C7H15NO |
| Molar mass | 129.22 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | sweet |
| Density | 0.93 g/cm3 |
| Solubility in water | soluble |
| log P | 0.55 |
| Vapor pressure | 0.0457 mmHg at 25 °C |
| Acidity (pKa) | pKa = 10.38 |
| Basicity (pKb) | 6.84 |
| Magnetic susceptibility (χ) | -59.37·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.468 |
| Viscosity | 1.1 mPa·s (at 20 °C) |
| Dipole moment | 2.33 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 353.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | '-229.7 kJ/mol' |
| Std enthalpy of combustion (ΔcH⦵298) | -3906.6 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | `GHS07` |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. H335: May cause respiratory irritation. |
| Precautionary statements | Precautionary statements: P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | > 93 °C |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 >2000 mg/kg |
| NIOSH | SAF89725 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg/m3 |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
1-Ethylpiperidine Piperidin-3-ol 1-Methylpiperidin-3-ol 1-Propylpiperidin-3-ol 1-Ethyl-4-hydroxypiperidine |