Chemists first began exploring the piperazine ring system for medical and industrial purposes before the Second World War. Back then, researchers saw piperazines as promising candidates for pharmaceuticals, and the main reason had to do with their ability to modify molecular properties sharply. As organic chemistry boomed in the 20th century, tweaks to the basic structure produced compounds like 1-ethylpiperazine. This derivative offered chemists a way to fine-tune bioactivity, solubility, and reactivity. Over decades, 1-ethylpiperazine expanded from the pages of synthetic methods into diverse roles across industries, showing the lasting value of even slight adjustments in organic molecules.
1-Ethylpiperazine stands out as an organic compound based on a six-membered piperazine ring with an ethyl group attached at the nitrogen in the first position. This small change delivers notable shifts in both its physical behavior and how it interacts with other chemical systems. Labs and manufacturers commonly use this building block to help bridge gaps in medicinal, agricultural, and materials chemistry. Its popularity in catalogues of specialty chemical suppliers reflects the growing interest in personalized drug and catalyst design, where off-the-shelf molecules no longer meet all the requirements.
At room temperature, 1-ethylpiperazine usually appears as a colorless to pale yellow liquid. A faint amine smell gives away its basic nature. You can measure its boiling point around 155-160°C under normal conditions. Thanks to the ethyl group, its hydrophobicity rises compared to regular piperazine, but the molecule still shows plenty of water solubility. Those making use of it notice it blends smoothly in common organic solvents such as ethanol, methanol, dichloromethane, and acetonitrile, simplifying purification and reaction setup. Chemically, its basic ring resists hydrolysis but opens up predictable reactions with alkyl halides, acid chlorides, and even some mild oxidizing agents.
Suppliers list 1-ethylpiperazine by its CAS number, 5308-25-8, and often ensure minimum purity levels above 98%. This level of quality matters in both research and production, since traces of other piperazines or water can skew analytic results or produce unwanted side products. Along with purity claims, technical data sheets mention water content, refractive index, and precise density at 20°C. Packaged in amber glass bottles or aluminum containers, the product must carry hazard details according to global transport and handling laws. Labels feature health warning pictograms and clear guidance on proper storage.
Most chemists prepare 1-ethylpiperazine by direct alkylation, treating piperazine with ethyl bromide or ethyl chloride under basic conditions. Solvents like ethanol or acetonitrile keep everything in solution, and sodium hydroxide or potassium carbonate soaks up the acid made during the process. After several hours of stirring and gentle heating, crude product forms, which technicians then purify through distillation or liquid-liquid extraction. With careful control, this method delivers good yields, but each batch still gets inspected using GC-MS or NMR to confirm both identity and purity.
The nitrogen atoms in 1-ethylpiperazine act as strong nucleophiles, opening doors to a wide range of functionalization strategies. Medicinal chemists rely on these positions to bolt on pharmacophores and tailor drug candidates. Reaction with acyl chlorides or sulfonyl chlorides forms stable amides or sulfonamides, central to both small molecule drug research and polymer design. Its secondary nitrogen also welcomes reaction with isocyanates, giving access to ureas. For those extending its utility, modifications such as alkylation at the free nitrogen or arylation turn 1-ethylpiperazine into new tools for ligand design and materials engineering.
The systematic name, 1-ethylpiperazine, sometimes takes a backseat to variations such as N-ethylpiperazine, 1-Et-piperazine, or N-ethyl-hexahydropyrazine. Chemical catalogs across regions use different identifiers—Germany lists it as N-Ethylpiperazin, while Japan refers to it by its Japanese Industrial Standard number. In research circles, the simplest shorthand, “EtPip”, crops up in lab notebooks and publications. Recognizing these names helps chemists track down reliable sources and avoid confusion in multi-lingual collaborations.
1-Ethylpiperazine carries both health and handling risks. Direct skin contact causes irritation, so users wear nitrile gloves and eye protection. Inhalation of its vapors leads to headache, nausea, or even more serious symptoms with heavy exposure. Labs use chemical fume hoods to keep air clean and storage lockers with ventilation to prevent buildup of vapors. Spills require neutralization with dilute acid and cleanup using absorbent materials rated for amines. Firms following REACH in Europe or OSHA in the United States train all handlers and regularly revisit safety data sheets.
Researchers and manufacturers apply 1-ethylpiperazine in many fields. Drug development teams incorporate it into candidate molecules aiming for improved solubility, lower toxicity, or enhanced receptor targeting. In agrochemicals, tweaking the piperazine ring can dial in the pest-fighting ability of active ingredients, making crops safer and yields higher. Water treatment specialists employ ether derivatives of this scaffold for chelation and removal of heavy metals. Industrial coatings and resins include its motifs for extra flexibility and toughness.
New findings keep shining light on the versatility of 1-ethylpiperazine. Labs worldwide experiment with ring-functionalized versions seeking breakthroughs in central nervous system drugs and cancer therapies. Collaborative teams in academia and industry tweak its structure to influence blood-brain barrier penetration and metabolic stability. Sometimes the push comes from sustainable chemistry, as greener synthesis and waste reduction take priority. R&D projects rely on both historic knowledge and recent computational models, showing that real advances need both experience and creative thinking.
Groups studying toxicity focus on acute and chronic effects. Rats exposed to high doses by inhalation or ingestion develop mild to moderate symptoms, but human data remains sparse. Most flagged issues relate to its strong basicity and potential to cause cell membrane disruption. Regulatory agencies call for clear documentation on dose-response and metabolism before granting free access or inclusion in consumer-facing products. Long-term trials exploring genotoxicity and carcinogenicity suggest moderate risk at occupational exposure levels, but proper engineering and personal protective standards keep incidents rare.
Growing demand for specialty molecules ensures 1-ethylpiperazine will stick around. As machine learning speeds up the search for new drugs, this class of compounds may see a surge in tailored modifications, leading to safer, more effective treatments. Environmental regulators press for low-impact synthesis routes, so chemists keep searching for catalysts and conditions that cut down on waste and risk. Any innovation that shapes a more precise, less polluting chemical industry will call on molecules like 1-ethylpiperazine for both building blocks and inspiration.
Walk into a chemical manufacturing lab or talk with anyone who deals with pharmaceutical ingredients, and sooner or later you'll hear the name 1-ethylpiperazine. This compound isn't a household item, but in specialized circles, people count on it for some important jobs. Like a surprising number of things hiding in chemical supply catalogs, its work ripples far beyond what you'd expect from a clear liquid in a flask.
Most chemists who work on drug discovery recognize 1-ethylpiperazine as a vital building block. Its role often starts and ends behind the scenes — think about medications like antihistamines, antidepressants, and anti-infectives. Some cancer drugs take shape around its piperazine ring. At this stage, it helps tweak how a molecule behaves inside the body, either by bending the way it binds to receptors, or by changing how long it stays active. No one swallows “1-ethylpiperazine tablets,” but people benefit from pills crafted using its foundation.
In chemical research, shortcuts save time, but quality matters more. This molecule helps chemists move from step to step by allowing quicker construction of complex structures. Because it offers a fairly reactive nitrogen atom, it slots right into syntheses that call for attaching other side chains or rings — like putting custom rims on a car. That flexibility means more researchers can experiment with new pharmaceuticals, and new products enter clinical trials sooner.
Drugmakers aren’t the only ones paying attention. The agrochemical industry also sees 1-ethylpiperazine as a solid teammate for making pesticides or herbicides, adding stability to products or boosting their effects. That crosses over into all sorts of sectors: a cotton farmer trying to keep pests away or a company testing new fungicides for greenhouses may benefit from products that had 1-ethylpiperazine in their recipe.
People often overlook raw chemical sourcing, but the quality of a compound like this shapes whether an end product works safely and effectively. Pharmaceutical companies run exhaustive purity checks and follow tight rules set by regulatory agencies. Even one tiny impurity changes the effectiveness of the medicine or triggers dangerous reactions. This pushes suppliers to keep standards high. A single misstep echoes all the way down to patients or consumers.
Many people think of lab chemicals as obscure, but a lot of real-world impact follows from choices made in supply chains. Health authorities such as the FDA and EMA monitor not just finished medicines, but also the raw building blocks, including 1-ethylpiperazine. Keeping supply chains transparent reduces counterfeiting, contamination, and illegal usage. I’ve seen regulations step in after contamination scares, and each time the response forced stricter traceability.
The world keeps pushing for greener chemistry. Safer handling, less toxic waste, and better worker protection spark innovation. Chemists keep searching for cleaner reactions that use the same key ingredients with less risk. In my early days at a chemical lab, we always ended safety meetings by comparing the old ways with new advances. Simple steps, like closed-system reactors and smart sensors, already help reduce accidents when handling chemicals like 1-ethylpiperazine.
1-Ethylpiperazine might not make headlines, but it shapes a host of products people use daily — sometimes lifesaving, sometimes necessary for growing food. Trust in these unseen ingredients starts with oversight and ends with research that doesn’t cut corners.
Every day, we count on chemistry more than most folks realize. Medications, plastics, cleaners—almost everything around us grew out of a handful of basic molecules. 1-Ethylpiperazine shows up in both industrial and research settings, showing the impact of such “niche” molecules outside of textbooks. The question about its formula isn’t just about memorizing letters and numbers. It’s about understanding how structure gives a molecule its function, and how that function can change lives.
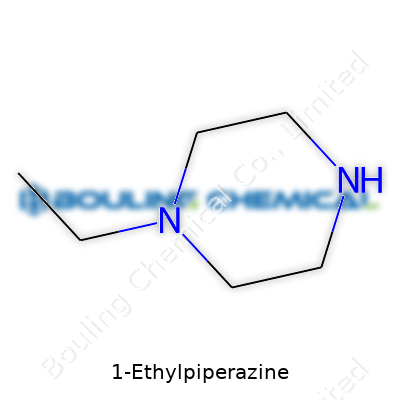
1-Ethylpiperazine is built from the piperazine ring—a six-membered ring made from four carbon atoms and two nitrogen atoms sitting across from each other. Piperazine itself fits into drug development and acts as a backbone for making new molecules. When you attach an ethyl group to the first nitrogen atom in that ring, you create 1-Ethylpiperazine. Its molecular formula is C6H14N2. On paper, it looks simple. In practice, just that small tweak in structure can change how it behaves in a chemical reaction or inside a human body.
Pharmaceutical research depends on compounds like this one. Medicinal chemists often use 1-Ethylpiperazine as a building block for bigger drugs. The ethyl group brings changes to the molecule’s shape, improving its solubility or tweaking how it interacts with drug targets. Anyone working in a chemistry lab learns quickly that small changes in structure can unlock new possibilities—or close doors just as fast. 1-Ethylpiperazine’s structure provides an easy platform for adding more groups, building molecules that treat disease or block infection.
Chemicals like 1-Ethylpiperazine can help, but they also require respect. Its formula may seem harmless, but certain piperazine derivatives carry toxicity hazards or trigger allergic reactions. Researchers rely on up-to-date safety sheets and protective gear. I remember my earliest lab work, where we cleaned up after a minor spill. Even well-understood chemicals can surprise you, reminding us to keep precautions in place. Safe handling supports healthy outcomes, whether in medicine or manufacturing.
Digging deeper into a formula like C6H14N2 demonstrates more than rote learning. Google’s E-E-A-T stands for experience, expertise, authoritativeness, and trustworthiness—they matter as much in chemistry as in news writing. People want reliable answers, straight from those with both knowledge and experience. I’ve seen firsthand how inaccurate answers can sidetrack students and professionals. Those in research or even casual learners should look for high-quality references: scientific journals, university databases, or trusted lab manuals share specifics clearly and responsibly.
More folks should know that a “chemical formula” carries meaning beyond symbols. 1-Ethylpiperazine’s C6H14N2 results from combining thoughtful science and practical experience. This highlights why foundational concepts endure. Taking care with formulas empowers better decision-making in both professional labs and community settings. Cutting corners—especially around safety or scientific rigor—leads to setbacks nobody wants. So each formula, as basic as it may seem, underpins modern advances across treatment, technology, and daily living.
1-Ethylpiperazine shows up more in labs than in everyday talk, but anyone working with chemicals or around manufacturing spaces should know a bit about this compound. It’s not just a name on a bottle. Under the right conditions, it can cause real trouble. Breathing in its vapors or letting it touch your skin could lead to nasty health effects, and that’s something we all want to avoid in any home or workplace.
Think about all the things you could buy at a hardware store: some look harmless, but they don’t come with a skull-and-crossbones on the label. 1-Ethylpiperazine usually ends up in industrial processes—making drugs, coatings, and other chemicals. Safety sheets list it as corrosive. Spilling some on your skin or splashing it in your eyes makes a painful memory. Getting a whiff of the vapor can mess with your respiratory system, causing coughing, sore throat, or worse. Working in those spaces, I learned to never cut corners around “low-key” chemicals.
It’s not just about acute reactions. Repeated exposure without good gear or ventilation seriously increases health risks. Chronic exposure can leave long-lasting marks—especially if workers don’t have proper gloves or masks. The way this substance penetrates the skin and travels through the body means small mistakes add up over weeks or months.
Agencies like the European Chemicals Agency (ECHA) mark 1-ethylpiperazine as hazardous. Studies highlight irritation to skin and eyes, along with trouble for airways after inhalation. Large doses in animal studies led to damage that most would call significant. Poison control databases list several cases of burns, rashes, or respiratory distress linked to handling this stuff without adequate protection.
Workplace reports draw direct lines between lax handling and health problems later. Even if you don’t notice symptoms right away, over time, small exposures stack up. The stories I’ve heard from shop floors or research labs put the science into sweat-and-blood reality. Folks know better gear and warnings lower injury rates, but some employers still cut costs—until someone ends up needing medical care.
Experience drives home one lesson: treat 1-ethylpiperazine with the same caution you would give stronger acids or bases. Always wear chemical-resistant gloves, goggles, and a lab coat. Work in well-ventilated rooms, with fume hoods whenever possible. Clean up any spills right away, using proper absorbents instead of makeshift towels or rags.
Label everything clearly. Make sure everyone in contact with chemicals gets solid training—not just a quick rundown but hands-on safety drills. Company leaders should schedule regular safety checks and stock up on emergency eyewash and showers. I’ve seen lives saved because someone acted fast, not because they waited for the paperwork to catch up.
Safe chemical handling starts long before opening the bottle. Learn the risks, have the right gear, and never shortcut safety. Good habits around 1-ethylpiperazine protect not only your own health but also coworkers, janitors, and anyone else sharing the air.
1-Ethylpiperazine looks like just another clear liquid in a lab, but anyone who’s spent time working with organic chemicals knows there’s more to safe storage than just a label on a bottle. This compound plays a role in pharmaceuticals and specialty chemicals, and mishandling it can cause problems both in terms of health and regulatory headaches. I’ve seen labs that treated chemical bottles as just “stuff to be tucked away” and later paid the price—literally and with emergency cleanups.
Many folks ignore temperature guidelines, thinking a basic shelf or a desk drawer will do the trick. That’s a dangerous shortcut, especially with chemicals like 1-Ethylpiperazine. Room temperature isn’t a fixed number, and anyone relying on “whatever the air feels like” takes a gamble. Most chemical data sheets recommend a storage range between 2°C and 8°C—or at least, a spot far from heat sources. I once visited a small facility where a hot pipe running behind a storage shelf was the culprit in repeated chemical deterioration. That lesson stuck. Heat does more than reduce shelf life; it turns volatile liquids like this one into a workplace hazard before you even realize it.
People love shortcuts. Sometimes, lids get left loose after a hurried day, or old containers become backup storage without a second thought. With 1-Ethylpiperazine, every exposed surface increases the chance of evaporation or contamination. A well-sealed, airtight container with a clear, chemical-resistant label solves more problems than most realize. Keeping water and oxygen away limits peroxide buildup and keeps the chemical stable. Extra care means fewer headaches later on, and I can recall colleagues who saved their departments the cost of full chemical disposal just by following this one habit.
Fumes hang in the air. Not everyone thinks twice about the faint smell when they walk into a storage room, but repeated low-level exposure isn’t healthy. Vapors from 1-Ethylpiperazine can create an unpleasant environment or, in higher concentrations, push air quality below safety limits. Storing chemicals in a well-ventilated, dedicated cabinet—or better, in a fume hood area—keeps these risks in check. More than one study has found that simple ventilation drops air contaminant levels well below OSHA and NIOSH limits, making the extra fan or exhaust system a worthwhile investment.
Regulatory compliance isn't just about paperwork. Inspections go badly once someone spots makeshift shelving, missing hazard symbols, or chemical leaks on floors. Insurance companies and fire marshals look for fireproof or chemically resistant cabinets—both for regulatory reasons and because they’ve seen what a leaking bottle can do. Storing 1-Ethylpiperazine away from acids, oxidizers, and heat sources guards against unwanted reactions. Many chemical supply companies offer guidance, sometimes even site visits; tapping into that advice can sidestep common mistakes.
It doesn’t take a deep-dive seminar to keep 1-Ethylpiperazine safe: dedicate a cool, ventilated spot, check your containers, and keep incompatibles apart. Educating staff, using up-to-date safety data sheets, and taking a few minutes for regular inspections saves money, time, and—most importantly—keeps people healthy. In the world I’ve seen, those who invest a little extra effort up front rarely get surprised by trouble later on.
1-Ethylpiperazine lands on the radar for plenty of people working in chemical labs, pharmaceuticals, and research groups. If someone asks how to get a hold of this compound, it’s smart to dig a little deeper before reaching for the wallet. A casual internet search will turn up lists of suppliers. That only tells part of the story. The real challenge centers on dealing with legal rules, lab safety, and quality.
Rules about purchasing chemicals like 1-Ethylpiperazine change from country to country. One government agency might label it as a precursor to controlled substances, another might call it a harmless intermediate. My work with chemical orders has meant sending detailed paperwork to make sure everything checked out. Regulatory bodies, including the DEA in the United States and the European Chemicals Agency, watch who moves certain chemicals—tracking for both public safety and national security. Anyone interested in purchasing should start by checking their local laws and restrictions.
All suppliers aren’t created equal. At the bench in the lab, the smallest impurity in a chemical can ruin an experiment or put someone in danger. Reliable suppliers trace their product origin, ship under proper conditions, and give a certificate of analysis with every bottle. Looking for membership in specialty chemical associations, or checking third-party reviews, weeds out the fly-by-night sellers. Don't get tempted by deep discounts from companies with shady backgrounds or no clear business address. I’ve never seen a low-budget shortcut end well in professional chemistry.
Nobody likes paperwork, but reputable suppliers always ask for proof about the intended use. Labs I’ve worked in must fill out end-user statements, provide business credentials, and sometimes share license details. Doing so builds trust both for the buyer and the provider. This doesn’t just protect the business—it keeps researchers out of trouble if questions come up during an audit. Skipping this step just opens the door to serious fines or legal headaches.
Bringing 1-Ethylpiperazine into the lab goes beyond the purchase. This chemical gives off an amine-like odor, irritates the skin, and needs to be stored in tight containers, away from heat. My early days in the lab taught me that mishandling even simple chemicals triggers accidents and lab shutdowns. Good practice means reading safety data sheets, training staff, and equipping workspaces with proper solvents, gloves, and eyewash stations.
Asking for 1-Ethylpiperazine means getting comfortable with regulations and paperwork. Sourcing from established chemical suppliers, not online auction sites or unknown retailers, limits risks. Understanding the intended use and storage requirements keeps everyone safer. In every order I’ve placed, following up with due diligence seemed basic, but it always paid off. If a supplier cuts corners or skips documentation, move on.
Buying specialty chemicals like 1-Ethylpiperazine isn’t just about price and shipping speed. It’s about protecting research, colleagues, and the wider community. The right approach asks for patience, checks credentials, and follows both the letter and the spirit of the law. Better to lose a day to paperwork than put an entire project at risk.
| Names | |
| Preferred IUPAC name | 1-ethylpiperazine |
| Other names |
1-Ethylpiperazine N-Ethylpiperazine Piperazine, 1-ethyl- 1-Ethyl-1,4-diazacyclohexane |
| Pronunciation | /ˈwʌn-ˈɛθ.ɪl.paɪˈpɛr.əˌziːn/ |
| Identifiers | |
| CAS Number | 5308-25-8 |
| Beilstein Reference | 1817451 |
| ChEBI | CHEBI:39746 |
| ChEMBL | CHEMBL46073 |
| ChemSpider | 8761 |
| DrugBank | DB08317 |
| ECHA InfoCard | 100.012.646 |
| EC Number | 202-091-5 |
| Gmelin Reference | 8285 |
| KEGG | C14451 |
| MeSH | D010005 |
| PubChem CID | 7130 |
| RTECS number | EW2975000 |
| UNII | 2BD3588SOZ |
| UN number | UN2381 |
| Properties | |
| Chemical formula | C6H14N2 |
| Molar mass | 114.19 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | amine-like |
| Density | 0.868 g/mL at 25 °C |
| Solubility in water | miscible |
| log P | 1.13 |
| Vapor pressure | 0.85 mmHg (at 25 °C) |
| Acidity (pKa) | 9.78 |
| Basicity (pKb) | 5.62 |
| Magnetic susceptibility (χ) | -68.2e-6 cm³/mol |
| Refractive index (nD) | 1.474 |
| Viscosity | 0.848 cP (25°C) |
| Dipole moment | 2.51 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 386.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -61.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4063.7 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. Harmful if inhaled. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H314 |
| Precautionary statements | P280, P305+P351+P338, P337+P313, P264, P302+P352, P332+P313 |
| NFPA 704 (fire diamond) | 2-3-0 |
| Flash point | 82 °C |
| Autoignition temperature | 335 °C |
| Explosive limits | 1.7-10.4% |
| Lethal dose or concentration | LD50 (oral, rat): 2,120 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 2100 mg/kg |
| NIOSH | SE1575000 |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 200-500 mg/L |
| IDLH (Immediate danger) | IDLH: 3,000 ppm |
| Related compounds | |
| Related compounds |
Piperazine 2-Methylpiperazine 1-Methylpiperazine 1-Phenylpiperazine |