Imidazole chemistry stretches back more than a century, stoking interest among chemists since imidazole’s discovery in 1858. Substituted imidazoles didn’t waste any time finding their way into the pharmaceutical and chemical industries, and 1-Ethylimidazole soon followed. The compound came about as researchers were exploring nitrogen heterocycles for their distinct reactivities and usefulness. Laboratories in Europe, especially Germany and Britain, began synthesizing alkylated imidazoles like 1-Ethylimidazole for their promise in both organic synthesis and as ligands in coordination chemistry. It is not an exaggeration to say a huge chunk of modern heterocyclic chemistry owes a nod to those early efforts.
1-Ethylimidazole looks like a clear, colorless to pale yellow liquid at room temperature, sporting a mild, amine-tinged aroma. Chemists recognize its value both as a building block and as a solvent or additive, capitalizing on its electron-rich imidazole backbone. Over time, it has carved out a valuable spot in labs and industry setups focused on synthesis, catalysis, polymers, and biological molecules. Researchers come back to it often, drawn by the simple modification of the imidazole family that brings the ethyl group to the N1 position—creating opportunities for tailored reactivity.
1-Ethylimidazole's molecular formula is C5H8N2, with a molecular weight coming in at 96.13 g/mol. It boasts a boiling point around 210°C and a melting point well below freezing, solidifying only around −55°C. Water solubility sits in the moderate-to-high zone, which allows straightforward handling in aqueous and organic syntheses. Thanks to the presence of two nitrogen atoms, it can act as a mild base and bind easily to metals in catalytic systems. The compound's volatility is low, which comes as a relief during scale-up or when security showers are a long walk away. One whiff of the liquid and most seasoned chemists recognize the familiar sharp tang of the imidazole family, laced with a whiff of bananas due to trace breakdown products.
Experienced laboratory staff keep their eyes peeled for labeling that clearly lists the compound’s concentration, purity (often above 98% in high-grade lots), and batch tracking numbers. Certificates must mention water content, usually kept under 0.5% to avoid compromising sensitive syntheses. Proper storage means airtight containers, away from flames or direct heat, as trace acids can prompt decomposition. Regulatory paperwork details UN or CAS numbering (CAS 1072-63-5), handy for cross-referencing and supply chain accuracy. Containers always warn about strong base or oxidizer incompatibility, sticking a big exclamation mark on safety data sheets when distributed for either academic or industrial purposes.
Seasoned chemists often prefer the N-alkylation of imidazole using ethyl halides. For bench-scale work, imidazole gets stirred up with ethyl bromide or ethyl iodide in a polar solvent, with a mild base to mop up the resulting hydrogen halide. A reflux process runs for several hours followed by vacuum distillation to catch the volatile product. Industrial producers streamline the process, sometimes swapping out solvents for greener alternatives and employing continuous reactors. Salt byproducts must be filtered and handled thoughtfully to avoid process headaches and environmental headaches down the line. Every operator worth their goggles knows clogging up a distillation line with stray salts can wreck productivity for days.
Having the ethyl group on the N1 position doesn’t stop the molecule from joining a host of organic transformations. Chemists love 1-Ethylimidazole for nucleophilic substitution and as a ligand in transition metal chemistry. It can participate in acylation and alkylation reactions without awkward side reactions that can plague other imidazole derivatives. The molecule can be quaternized, oxidized or turned into ionic liquids, giving it access to modern applications like catalysis in green chemistry or advanced battery technology. Researchers have found that minor swaps—like changing the ethyl for a bulkier group or swapping ring nitrogens—lead to markedly different biological and physical properties.
Walk into any reasonable-sized chemical storeroom and you’ll find 1-Ethylimidazole labeled as N-Ethylimidazole, 1-Ethyl-1H-imidazole, or just Ethylimidazole in older records. Chemical catalogues list it under CAS 1072-63-5, easing international procurement hassles. A few specialty suppliers might call it Imidazole ethyl derivative, though most reputable brands keep with current IUPAC naming for consistency.
Handling policies drill home the need for nitrile gloves and splash-proof eyewear. The compound can irritate skin and mucosa, and a quick splash in the eye can be an all-morning affair at the eyewash station. Ventilation, fume hoods, and closed transfer systems keep airborne exposure down. Material safety data sheets lay out procedures for spill containment—absorbers for small spills, evacuation plans for anything larger. Waste containers used for 1-Ethylimidazole need to be clearly labeled and kept apart from strong bases, acids, or oxidizers. Chemical hygiene plans in research facilities and production sites always keep 1-Ethylimidazole on the list of compounds requiring clear protocols and safety drills. Fire risk runs low, but not zero.
1-Ethylimidazole crops up everywhere from pharma research to electrochemical devices. Synthetic chemists lean heavily on it as a nucleophilic catalyst, while biochemists have explored derivatives as enzyme inhibitors and probes in protein labeling. Polymer scientists add it to improve flame retardancy or as a precursor for imidazole-based polymers, which find a home in special specialty coatings, adhesives, and membranes. Electrochemistry teams test it for use in ionic liquids, chasing after higher conductivity and thermal stability in batteries. Researchers in environmental science tinker with 1-Ethylimidazole-based frameworks for water purification and catalysis. I've seen industrial troubleshooting sessions pinpoint improvements in reaction selectivity just by tweaking the substitution pattern on the imidazole core.
Academic and industrial teams push for novel synthesis routes that cut down waste or costs, searching for greener ways to make 1-Ethylimidazole. Automation now lets companies run hundreds of reaction optimizations in parallel, shrinking time from idea to insight. Updates in analytical chemistry like NMR and HPLC let teams chase down trace impurities or side products, making it possible for every batch to meet exact purity specs. New projects look at chiral modifications, aiming to open up antibacterial or antifungal spaces left untouched by current drugs. Start-ups sniff around imidazole derivatives for use in next-gen batteries, convinced that the core can outperform older generations of ionic liquids. University chemists run old reactions in fresh ways, finding that subtle tweaks in reagents or reaction time can unlock selectivities that were previously out of reach.
Early animal studies show 1-Ethylimidazole carries low acute toxicity, though repeated exposure can stress the liver and central nervous system. Its structure lets it slip into biological systems, so researchers monitor metabolic byproducts for both safety and drug design. Workplaces with regular exposure install air monitoring and periodic blood work for staff, based on studies tracing metabolites in urine. The compound shows low mutagenicity in standard assays but isn’t completely benign—chronic high exposure can lead to nervousness, appetite suppression, or in worst cases, mild liver fibrosis, according to multi-week rodent studies. Teams balancing risk lean toward substitution with less bioavailable derivatives or keep strict exposure controls in place, especially for scale-up batches in crowded production spaces.
One look at the pipeline and it’s clear the story of 1-Ethylimidazole is just getting started. Synthetic chemists investigate new functionalizations to tap into pharmaceuticals with better selectivity and fewer side effects. Energy researchers stretch its promise as a core of ionic liquids, supporting advanced batteries or hydrogen storage systems. Environmental engineers play with 1-Ethylimidazole frameworks to catch heavy metals or break down persistent organic pollutants. Even biologists are returning to it, exploring the core's potential as a starting point for enzyme mimics or as a backbone for small-molecule sensors. As automation lowers the cost of screening and custom modification, expect 1-Ethylimidazole to pop up in more areas, especially those chasing safer, greener, and more efficient chemistry.
Scrolling through the world of industrial chemicals, you might miss 1-ethylimidazole among the titans of science. This little compound punches above its weight in the labs and factories where so many of our modern conveniences begin. It isn’t something people ask for at the hardware store, but chemists and manufacturers know it well for its usefulness.
I’ve spent my fair share of time troubleshooting dye reactions in a textile plant, and the right solvent can make everyone’s life a lot easier. 1-Ethylimidazole stands out for dissolving other substances where water or simple alcohols fail. It keeps reactions tidy and controllable, especially when making specialty polymers and coatings. In coatings production, a steady, reliable medium like this helps avoid ruined batches. If you’re working with stubborn pigments or tricky resins, you need a compound that doesn’t get overwhelmed easily.
Drug synthesis is expensive and delicate. In my previous job at a pharmaceutical R&D firm, folks sometimes hit a dead end looking for more efficient or cleaner reactions. 1-Ethylimidazole serves as a catalyst, speeding up steps that would otherwise drag on. Its structure lets it shuffle molecules into place, making it valuable for selective modifications of drug candidates—think of it like a subtle but highly skilled hand guiding traffic at a crowded intersection. This lowers waste and saves both energy and raw materials. For smaller producers, these savings mean the difference between an experiment stalling out and a breakthrough drug reaching the next phase.
Not every chemical gets to play a part in the shift away from fossil fuels. This one does. It acts as an electrolyte component for new battery designs, such as lithium-ion or flow batteries. I saw engineers at a research center blend compounds like this to chase longer battery lifespans and safer operation. With a stable electrolyte, batteries can charge faster and resist failure—big deals for electric vehicles and portable electronics. Compared to more toxic options, its safety profile also means fewer headaches during both handling and disposal.
Even versatile ingredients come with baggage. Handling chemicals like 1-ethylimidazole calls for care: it can irritate skin and eyes, and too much exposure may harm the lungs. I once watched an incident unfold where poor ventilation led to unnecessary panic and loss of production time. Training, good ventilation, and solid containment keep risks at bay, but cost-cutting can undermine those safeguards. Efforts to build safer production spaces, with better air filtration and protective equipment, mean workers stay healthy and focused.
As science races forward, demand for chemicals that tick multiple boxes—safe, effective, and adaptable—only grows. 1-Ethylimidazole plays a small part in the big machinery of progress, from medicine to devices people use every day. By supporting research into greener synthesis methods and reusable solvents, companies can lower their footprint and improve working conditions for everyone in the supply chain.
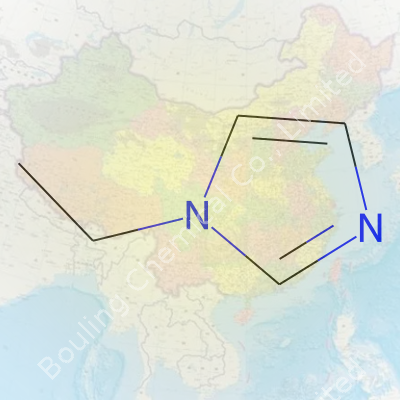
Ask a group of chemists about 1-Ethylimidazole and you’ll spot an immediate recognition. But out beyond the chemistry labs, this molecule rarely gets much attention. For clarity: 1-Ethylimidazole carries the chemical formula C5H8N2. Picture it as an imidazole ring — that classic five-membered loop of three carbons and two nitrogens at the 1 and 3 positions — with an ethyl group snugged onto the nitrogen at the first position. This small tweak to imidazole’s plain structure turns out to be a big deal for chemists, especially in pharmaceuticals and as specialty solvents.
I spent many hours in the lab, fiddling with molecules just like 1-Ethylimidazole. The ethyl group may look modest, but those two carbons make this molecule less sticky with water and give it a nudge toward dissolving in organic solvents. That changes up the whole game in synthesis work. Sometimes you need just enough solubility to get a catalyst to behave, or you’re after a reaction resistant to hydrolysis. The nature of the imidazole ring — aromatic, balanced, with nitrogen pairs positioned for hydrogen bonding or electronic tweaks — offers a versatile backbone chemists lean on all the time.
1-Ethylimidazole doesn’t fill the shelves of grocery stores, but you’ll find it in pharmaceutical pilot plants, specialized labs, and in some cases, as a precursor to more complex agents. Medicinal chemists value it for the same reasons I did: simple adjustments on this backbone let you nudge drug candidates to improve solubility or binding in a protein pocket. This is not just theory — plenty of antifungal drugs feature imidazole rings, and the ethyl addition is one more knob to twist in the hunt for better medicines.
With every new industrial or laboratory chemical, questions pile up. What happens if this leaks into water? Imidazoles aren’t as notorious as other chemical classes for causing harm, but neglecting environmental impact keeps biting us. 1-Ethylimidazole, due to its small size and moderate polarity, won’t hang around as long as some chlorinated solvents, but that doesn’t let anyone off the hook. Smart waste management, plus more research into biodegradability, ought to stay front and center.
Too many times I’ve watched labs rinse reaction flasks with chemicals no one wants to see outside a controlled environment. Newer chemists and manufacturers can lead on this, swapping out harsher solvents for molecules like 1-Ethylimidazole in certain conditions. But this only works if the supply chain values safety just as much as cost. Pressure for cleaner options must come from all sides — buyers, manufacturers, regulators — or else habits stick. Real progress happens one bottle at a time.
Next time someone points out a chemical formula like C5H8N2, remember that each piece of the structure carries a story. The way 1-Ethylimidazole bends, dissolves, and reacts isn’t just an abstract concept. These details set the tone for entire research programs, influence waste habits, and quietly shape the choices made in science every day. If more people, not just chemists, looked beyond the formula, we would probably wind up making smarter choices about which molecules we keep close — and which we let go.
A bottle of 1-ethylimidazole in a chemistry lab doesn’t attract much attention, yet this single compound ends up shaping the outcome of a lot of experiments and processes. Anyone used to lab work knows: chemistry rewards careful habits and punishes carelessness. 1-ethylimidazole acts as a base, a solvent, and shows up in a handful of synthesis steps. It’s often flammable, can release irritating fumes, and doesn’t play well with moisture or high heat. Miss a step in its storage, and it’s not just the results you risk; it’s safety, equipment, and in some cases, people's health.
I once saw a promising experiment fail because someone left 1-ethylimidazole sitting on a sunny windowsill. Sunlight and warmth turned an otherwise pure compound into something messy and less predictable. Many chemicals react or break down when kept warm or exposed to light. 1-ethylimidazole is no different. Its boiling point sits below 200°C, but long before that, it starts to turn, darken, or even evaporate. Keep it somewhere cool and shaded, away from windows and heat lamps, and you avoid all kinds of headaches.
Nobody likes fumbling with stubborn bottle caps, yet a loose seal lets these chemicals slip out and fill the room. 1-ethylimidazole, left open or capped poorly, can stink up a whole workspace. Those vapors aren’t just annoying — they affect health, notebooks, even the sensitive equipment some labs rely on. A good, solid screw-cap keeps the liquid inside where it belongs. I always double check before leaving for the day, not just for myself but for everyone who uses that space after me.
Ask anyone who’s mixed this stuff with water by accident — the results aren’t pleasant. 1-ethylimidazole grabs onto moisture from the air, which can start unwanted reactions or change its strength. That ruins its usefulness and, in rare cases, causes pressure buildup if water sneaks into a sealed bottle. I found it smarter to store these chemicals along with other moisture-sensitive reagents, far away from sinks, humid benches, or spots prone to spills. Think dry and sealed every time.
I learned the hard way that mixing incompatible chemicals in a cabinet sometimes causes more trouble than storing them poorly. Acids and bases — 1-ethylimidazole leans on the basic side — don’t belong next to each other. Unexpected reactions or gas production could force open a cap or even shatter a container. Create distance from acids and oxidizers. Sticking to this simple rule spares everyone a mountain of paperwork and potential injury.
Nobody should have to guess about what’s in a bottle, how long it’s sat there, or whether it’s mixed with anything else. An accurate, visible label showing the date, contents, and full hazards gives new lab members or tired co-workers the push to handle things properly. If a bottle seems off or the liquid's changed color, it’s worth investigating before opening.
The best storage system for 1-ethylimidazole comes down to small habits. Close the bottle right after use, shield it from unnecessary light, and note every detail on the label. Store it high enough so leaks don’t drip on skin or onto electrical outlets. Make a habit of checking for leaks or pressure each week, especially in older bottles. One quick inspection saves far more hassle than any rushed shortcut ever could.
1-Ethylimidazole shows up in chemical labs as a solvent, a starting material, and occasionally as a specialty building block for medicinal chemistry. Most folks outside a research group rarely run into it, but for anyone handling bottles and pipettes, the label on the glass means something: risks sit in the mix along with its molecular structure.
Detailed material safety data sheets flag 1-ethylimidazole for irritation if it hits the skin, splashes in the eyes, or if its fumes get inhaled. Researchers from the National Institutes of Health and the European Chemicals Agency agree skin and eye irritation appear common. Breathe in the vapors, nose and throat start to feel it. Direct spills on the skin tend to cause redness or even a burning feeling.
Peer-reviewed reports do not show 1-ethylimidazole as a heavy hitter like benzene or formaldehyde—no strong evidence of cancer risks, teratogenic effects, or genetic harm in standard tests. Still, absence of long-term data is no reason for careless habits. Most chemical safety always falls back to “assume some risks, use gloves and goggles, work with good ventilation.”
In my years working next to benches and fume hoods, I’ve learned most injuries seem minor—until sniffles and rashes turn into something bigger. Eye rinses and skin washes happen more often than outsiders think, usually because a labmate figures “nothing too bad in here.” Yet, underestimating imidazoles and similar reagents leads to little cuts, slow burn reactions, or even a ruined afternoon tracking down stray fumes. Strewn gloves and a spill kit save you real trouble.
Smaller hazards often get overlooked, especially if no strong smell warns you. 1-ethylimidazole can sneak out as a vapor, and with repeated exposure folks may even develop sensitivity, leading to chronic symptoms. Pay attention to the wisdom in old lab habits—many of us remember at least one case of a “minor” irritant causing trouble for weeks.
Focusing on practical solutions, ordinary steps go far. Store it tightly sealed, away from acids and oxidizers. A rubber stopper or Parafilm never hurts in a busy lab fridge. Wearing gloves isn’t just about protecting yourself; it avoids contaminating everything from your notebook to your lunch sandwich. Proper fume hoods and eye shields handle the rest. If a spill happens, absorb it with inert material and toss it in the designated waste—no shortcuts, no pouring down the drain.
Some colleagues like to test detection with litmus paper or other quick tricks, but real safety means keeping a careful inventory—know the difference between a full bottle and one that’s starting to make your eyes water as soon as you flip the cap. Rely on training refreshers; the stories from folks who ran into problems stick longer than any printed warning.
Few outside chemistry circles want to think about routine chemical handling. Yet, stories about accidents sometimes hit headlines, making all chemical work seem mysterious or dangerous. Honestly, your coffee pot runs more risk of causing long-term health issues than a properly handled vial of 1-ethylimidazole. Still, respect for risk helps everyone involved in science—students, seasoned techs, and even school interns—make it home with nothing worse than a sharp memory.
1-ethylimidazole presents hazards you can predict and avoid. Treat it with reasonable respect, use good habits, and keep common sense handy; you’ll avoid most trouble before it ever has a chance to start.
Anytime you walk through a lab, there’s a certain look in people’s eyes when someone mentions “purity.” No matter what corner of chemistry you work in, purity tells you what you can trust out of a bottle. For 1-ethylimidazole, this rings especially true since a tiny slip in purity can throw off whole experiments or industrial runs. That isn’t just chemist paranoia talking. A random contaminant could turn an easy reaction into a frustrating mess or send a process into full shutdown if you scale things up.
You’ll see two main types of 1-ethylimidazole grades getting tossed around: “reagent grade” and “industrial grade.” For the lab bench, the cleaner, higher-purity kind is the one labeled “reagent grade.” Purity here often zooms in at 98% and up—sometimes advertised as 99% or even a decimal point higher for nerve-wracking syntheses. You’ll see these used in pharmaceuticals, material science trials, or in settings where trace impurities have unpredictable effects. This is where accuracy beats price, and chemical noise isn’t tolerated.
Industrial grade sits at a lower purity range, usually dipping below 98%. This isn’t some cost-cutting shortcut. Bulk manufactures and bigger chemical plants often plan for downstream purification or can carry impurities through steps that won’t matter for their final product—or they’re making solvents where “close enough” works. Sometimes you get manufacturers who won’t even bother listing impurities unless it’s something that’ll definitely interfere with big-picture processes. I’ve seen this cause headaches when someone forgets to check what’s actually in their drum and assumes it’s all clean.
Some producers go all out and specify how they check their purity. You see techniques like gas chromatography, NMR, or mass spectrometry giving buyers a sense of what else is lurking. That bit of transparency counts, especially since the tiniest traces of water or leftover reactants can spell disaster in certain syntheses. One supplier’s 98% might still have a completely different impurity profile from another. I’ve learned not to skip over those tiny details when someone’s in the middle of a sensitive project.
It’s not just academic; the stakes are real when you think about what goes wrong if someone uses off-spec 1-ethylimidazole. A run-of-the-mill impurity can end up as a catalyst poison, skew NMR readings, or ruin attempts at reproducibility. There isn’t a one-size-fits-all answer for the right grade. What’s needed for a proof-of-concept in the university lab just won’t cut it for a commercial pharmaceutical plant pushing regulatory standards.
Problems really show up when supply chains get tight or manufacturers play games with spec sheets. Over the last few years, more people started asking for certificates of analysis before they sign off on a shipment. I’ve seen better results when the whole team sits down and agrees exactly what grade matches each step in their work. Sometimes, extra purification in-house is a lifesaver. Sometimes, you need to bite the bullet and pay for top-tier purity just so everything downstream works out.
There’s a steady push for cleaner, more rigorously documented 1-ethylimidazole supply lines. Open communication between chemists and suppliers prevents mixing up grades and keeps surprises to a minimum. In the end, it’s about more than chemical formulas; it’s about making sure every reaction has the best shot at working, every single time.

| Names | |
| Preferred IUPAC name | 1-ethyl-1H-imidazole |
| Other names |
1-Ethyl-1H-imidazole Ethylimidazole |
| Pronunciation | /ˈwʌn ˌiːθɪl ɪˈmɪdəˌzoʊl/ |
| Identifiers | |
| CAS Number | 709-07-3 |
| 3D model (JSmol) | `/sitiojsmol/JSmol-2.7.4/JSmol.min.js?model=CCn1cncc1` |
| Beilstein Reference | 120924 |
| ChEBI | CHEBI:139427 |
| ChEMBL | CHEMBL1231784 |
| ChemSpider | 78627 |
| DrugBank | DB02137 |
| ECHA InfoCard | ECHA InfoCard: 100.018.987 |
| EC Number | 206-493-5 |
| Gmelin Reference | 67672 |
| KEGG | C07463 |
| MeSH | D017967 |
| PubChem CID | 7000 |
| RTECS number | NR8750000 |
| UNII | TWM1BB16JJ |
| UN number | UN2810 |
| Properties | |
| Chemical formula | C5H8N2 |
| Molar mass | 110.15 g/mol |
| Appearance | Colorless to light yellow liquid |
| Odor | amine-like |
| Density | 1.029 g/mL at 25 °C (lit.) |
| Solubility in water | miscible |
| log P | 0.02 |
| Vapor pressure | 0.38 mmHg (25 °C) |
| Acidity (pKa) | 6.9 |
| Basicity (pKb) | 6.96 |
| Magnetic susceptibility (χ) | -58.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.485 |
| Viscosity | 2.23 mPa·s (25 °C) |
| Dipole moment | 1.89 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 181.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -11.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3235 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P264, P280, P302+P352, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-2-0-تر |
| Flash point | 42 °C |
| Autoignition temperature | 455°C |
| Explosive limits | Explosive limits: 2.3–15% |
| Lethal dose or concentration | LD50 oral rat 970 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 775 mg/kg |
| NIOSH | NIOSH: KZ3850000 |
| REL (Recommended) | 1.5 mg/L |
| Related compounds | |
| Related compounds |
Imidazole 2-Methylimidazole 4-Methylimidazole 1-Methylimidazole 1-Butylimidazole |