Looking back through the annals of synthetic chemistry, many pyrrolidine derivatives took center stage during the wave of organic innovations in the latter half of the twentieth century. 1-Ethyl-2-(Nitromethylene)Pyrrolidine arrived on the scene as researchers experimented with pyrrolidine rings, seeking routes toward more reactive, selective intermediates for pharmaceuticals and advanced materials. The journey drew upon early nitroalkene chemistry, where the nitromethylene group stood out for both its engaging reactivity and versatility. Over the decades, chemists mapped out practical synthesis routes and unravelled the mechanistic backbone, fueling a modest but steady rise in interest, especially as nitroalkene chemistry found fresh applications across medicinal and catalytic research. Work by European research groups in the late 1980s highlighted the potential for targeted modifications, drawing sustained attention from laboratories working on heterocyclic scaffolds.
1-Ethyl-2-(Nitromethylene)Pyrrolidine stands as a solid example of how chemists have harnessed heterocyclic frameworks to unlock new synthetic possibilities. At its core, the compound brings together a stable pyrrolidine ring and a provocative nitromethylene group. The nitromethylene, linked to position 2 of the nitrogen-containing ring, doesn’t just sit quietly; it commands attention with strong electron-withdrawing nature, making the molecule more reactive when paired with nucleophiles and functional group transformations. This combination allows the compound to serve as both an intermediate in multi-step syntheses and a functional additive in specific research contexts, with structure–activity relationship studies showcasing the unique edge provided by the ethyl group at the nitrogen atom. Practical shelf stability, predictable behavior in reaction setups, and a distinct profile in chemical libraries round out its appeal in research settings.
With a melting point usually hovering around room temperature, 1-Ethyl-2-(Nitromethylene)Pyrrolidine generally appears as a crystalline powder or low-melting solid. The color ranges from pale yellow to off-white—a clear signal of nitro group presence. The compound emits a slight amine-like odor, a reminder of its pyrrolidine backbone. Solubility sets the stage for practical applications: it dissolves readily in polar organic solvents such as ethanol, acetone, and dimethyl sulfoxide, yet shows limited solubility in water, ensuring it can be manipulated in both analytic and preparative set-ups without fuss. The nitromethylene group shows up in UV-vis and NMR spectra, offering distinctive peaks, while thermal gravimetric analysis confirms that the material remains stable well above ambient temperatures, usually up to 140°C before decomposition sets in.
Standard vials on the research market often carry labels noting high-purity grades (98% or better) suitable for analytic and synthetic purposes. In line with international chemical trade practices, labels include the molecular formula (C7H12N2O2), batch number, date of manufacture, storage instructions (room temperature in dry conditions), and warnings tied to irritant and oxidizing characteristics. In advanced research settings, certificates of analysis document spectral purity, elemental analysis, and residual solvent data. Handling instructions often recommend working under inert gas during larger-scale reactions, as well as using certified personal protective equipment (PPE) during sampling and transfer operations.
To make 1-Ethyl-2-(Nitromethylene)Pyrrolidine, skilled hands typically start with the carefully protected pyrrolidine ring. The synthesis usually kicks off with N-ethylation of pyrrolidine using ethyl bromide in the presence of a basic catalyst such as potassium carbonate. Once the nitrogen position sports its ethyl group, a formylation step offers a route to introduce the necessary methylene group at the 2-position. With this in place, nitration via a Vilsmeier-Haack or nitromethane-based protocol locks the nitromethylene group where it needs to be. Final purification steps rely on recrystallization from dry ethanol or careful chromatography, depending on impurities and batch scale. Keeping an eye on moisture during the nitromethylenation step improves yield and avoids side reactions, a lesson most synthetic chemists learn with experience.
Expect the nitromethylene group to drive much of the action in reactivity studies. Under basic, nucleophilic assault, the nitromethylene undergoes addition and condensation, paving the way for Michael-type reactions, reductive transformations, and even cycloaddition with electron-rich alkene or diene partners. Hydrogenation over standard Pd/C catalysts pulls the nitro group down to various amine derivatives, which opens new doors for bioactive scaffold development. In oxidative settings, the ethyl group remains inert, making the molecule more predictable across a wide range of synthetic conditions. Functionalizations at the 3- or 4-positions of the pyrrolidine ring, through either halogenation or alkylation, broaden the chemist’s toolkit, allowing construction of libraries for SAR studies or ligand discovery in catalysis.
In catalogues and patents, 1-Ethyl-2-(Nitromethylene)Pyrrolidine may pop up under alternative monikers. “N-Ethyl-pyrrolidine-2-nitromethylene,” “Ethylpyrrolidine nitromethylene derivative,” and even shorthand notations such as “ENM-Pyrrolidine” show up. While the IUPAC format holds court in research publications, commercial suppliers sometimes create shorter trade names for clarity. These names, though simplified, often tie directly to laboratory order codes for easier logistics and minimized confusion during procurement.
Working with nitroalkene compounds calls for respect and diligence. 1-Ethyl-2-(Nitromethylene)Pyrrolidine may trigger mild to moderate irritation on contact with skin or eyes, so nitrile or latex gloves and splash-proof goggles become part of the routine. Adequate ventilation matters; working in a chemical fume hood keeps inhalation risks on the safe side, especially during experimental scaling or solvent evaporation. Spilled compound on benches or glassware cleans up with isopropanol followed by detergent. Disposal needs follow local regulations for nitro-containing organics, usually through approved chemical waste programs. Regular audits and annual refresher training help research teams stay sharp and keep workplaces safe.
Chemists exploring new drug candidates sometimes reach for this nitromethylene-pyrrolidine as a platform for anti-infective or neuroactive lead development. The combination of a flexible, basic nitrogen atom (from the pyrrolidine) and a highly reactive nitromethylene group attracts interest from teams searching for compounds that disrupt pathogenic processes at the molecular level. Catalysis also benefits; the strong electron pull from the nitroalkene segment modifies the behavior of metal complexes and organic catalysts in both experimental and pre-commercial settings. Some scientists find value in using this scaffold as a ligand precursor or in preparing custom monomers for functional polymer research, seeking novel material properties by tuning substituent patterns around the pyrrolidine ring.
Academic and corporate laboratories keep exploring the possibilities of 1-Ethyl-2-(Nitromethylene)Pyrrolidine, both as a synthetic intermediate and as a lead in biological screens. Over the last decade, computational modeling married with high-throughput chemistry has mapped out plausible derivatives, pointing researchers toward rings with enhanced selectivity or bioavailability for medicinal chemistry projects. Synthetic modifications—expanding core fragments, swapping functional handles, or branching alkyl chains—showcase just how far this core structure can stretch. Publications highlight not only synthetic routes but also mechanistic studies, providing better understanding of reaction pathways and opening new fields for structure–activity investigations in the search for next-generation therapeutics.
Experience with similar nitroalkene and heterocyclic compounds suggests that careful management of exposure matters. Researchers measure acute toxicity in standard laboratory animals, finding mild to moderate systemic effects at relatively high doses, mostly involving transient central nervous system symptoms and mild hepatic enzyme changes. In vitro studies using cultured hepatocytes and classic Ames tests scan for genotoxicity, with current data pointing to low mutagenic risk for this specific compound. Chronic exposure scenarios rarely arise, yet prudence would dictate periodic health monitoring for laboratory staff handling larger quantities. Safety data sheets remain essential reading, offering guidance on first aid procedures and providing context for interpreting rare but possible adverse reactions.
Looking out over the horizon, 1-Ethyl-2-(Nitromethylene)Pyrrolidine continues to sit at a valuable intersection of synthetic practicality and functional promise. As interest grows in green and sustainable chemistry, researchers may unlock more mild, eco-friendly syntheses, reducing waste and improving yield without compromising versatility. Drug discovery teams persist in searching for small molecules that strike the right balance of bioactivity, selectivity, and safety, and the flexible chemistry of this compound fits that brief. Advances in catalyst design—using pyrrolidine-based ligands—could rely on customized nitromethylene analogues, tapping the electron-pushing properties baked into this structure. While regulatory landscapes may place new demands on safety and documentation, the compound’s enduring adaptability keeps it on the radar for teams searching for better, faster, and smarter ways to solve today’s chemical challenges.
Anyone who's looked at the world of new and obscure compounds knows there's usually more than meets the eye. 1-Ethyl-2-(Nitromethylene)Pyrrolidine falls right into that camp. Scientists and law enforcement have both paid attention, but for different reasons. On the surface, this molecule isn’t sitting on a drugstore shelf or helping cure cancer. Instead, it pops up in labs, sometimes in legal grey areas, often linked to attempts to stay one step ahead of drug bans.
I remember back in college, folks went searching for legal highs as soon as regulations hit common substances. Chemists work relentlessly to tweak molecular structures, looking for fresh compounds with similar effects but no legal history. That’s the world this pyrrolidine-based compound comes from. People with a technical background saw potential in its skeleton, mostly as a stepping stone rather than a finished product. It bears similarities to some controlled substances used for illegal drug synthesis, particularly synthetic stimulation drugs.
Regulators have struggled to keep up. The DEA and others track new chemicals that appear in illicit labs. 1-Ethyl-2-(Nitromethylene)Pyrrolidine showed up during investigations of synthetic cathinones and stimulants—compounds that mimic the effects of things like amphetamines. Instead of targeting well-known drugs, chemists focus on relatives just a few atoms different. The law often lags behind, so these structures sometimes fly under the radar. Recently, labs test for its fingerprints because it signals possible illegal manufacturing, not because it carries a direct benefit like an over-the-counter medication.
Most mainstream industries have no everyday need for this molecule. It doesn’t feature in paint, plastics, or pharma research in any significant way—at least not as an end product. If you see it mentioned in scientific literature, the context is normally forensic. Researchers trying to map out how synthetic drugs mutate commonly reference this structure as a precursor, not a final goal. My own search for above-board, positive applications has turned up nearly empty. That lack of beneficial use tells a story. If a compound mostly lives in the shadows, lawmakers pay extra attention.
There’s real tension between scientific creativity and the harm that can come out of it. I get the appeal of pushing chemical boundaries, but some molecules like this one seem custom-designed for loopholes. Legitimate research has suffered from overbroad bans, which can chill innovation. At the same time, leaving such chemicals unchecked creates a playground for bad actors. Agencies have pushed for fast-track regulation tools, trying to respond in months instead of years. I’ve spoken to both chemists and public health officials—nobody’s totally satisfied with current policy, but everyone agrees on the stakes.
Education stands out as the most effective solution. Chemists, both inside and outside academia, need clear guidance about the forensic footprint of their work. Regulators, on the flip side, do best with strong scientific advice. Better collaboration would help both sides spot trouble earlier, instead of reacting late. Tracking these “grey zone” compounds, like 1-Ethyl-2-(Nitromethylene)Pyrrolidine, should stay a priority for anyone worrying about synthetic drug trade. Awareness and quick reporting, not just more rules, drive meaningful change.
1-Ethyl-2-(Nitromethylene)Pyrrolidine stands out in the lab for its potential hazards. Recent studies point to its sensitivity to heat and shock. For anyone who's worked with similar nitro compounds, the risk of sudden decomposition or fire can't be ignored. This isn’t just a rulebook issue—it’s about protecting yourself, colleagues, and your space from real danger. I remember one team member losing a fingernail after a small-scale spill went sideways. Cheap gloves and inattention did the damage.
Direct contact with this compound can irritate skin, eyes, and lungs. Wearing fitted nitrile gloves, splash-resistant goggles, and a lab coat gives a real layer of protection. It pays to swap out gloves regularly and double-up if there's a risk of splash or spray. Respiratory protection can’t be skipped in poorly-ventilated rooms—one whiff of a volatile nitro goes straight to your head. Fume hoods should run on high, not just for show, and it makes sense to keep the workspace tidy so spills and accidents don’t get compounded by clutter.
Take stock of its instability above room temperature and in direct light. Any chemist who’s seen a yellowed bottle from a sunlit bench knows the warning signs. Store it dry, cool, and away from ignition sources. Explosion-proof refrigeration beats open shelving. I’ve made the mistake of shelving unstable materials too close to oxidizers—one bad day wipes out months of careful work. Secure containers help, with labels marked clearly for emergency workers. Some labs use locked cabinets to be sure nobody stumbles into a pile of trouble.
Spills command immediate attention. Friend of mine learned this the expensive way—corrosive nitro solution soaked into a desk and left a mark that never truly left. Absorb small spills with vermiculite or sand before bagging for hazardous waste pickup. Never, ever flush this stuff down the sink. That’s an environmental incident waiting to happen. Stick to approved hazardous waste protocols, and always let the safety team know about incidents. Reporting isn’t about punishment—it’s about keeping everyone in the loop so bigger problems don’t sneak up later.
Some researchers try to run a prep solo, thinking quiet hours mean less distraction. But if things go wrong, isolation buys no time for help. Always let a labmate know what you’re doing and keep emergency contact info posted right at hand. Keep fire extinguishers, spill kits, and eye-wash stations unblocked and clearly marked. The worst thing after an accident is fumbling through clutter or scouring for the safety shower handle.
Training pays off more than any fancy equipment. The trend toward regular safety refreshers at universities comes from the aftermath of too many close calls. Sharing information openly about incidents, using real examples instead of theoretical what-ifs, gets the point across. SOPs (standard operating procedures) written in plain language, plus peer reviews before a big synthesis, create real barriers between a productive project and a bad news day. For anyone working with nasty compounds, double-check every step, and never skip the basics thinking experience alone keeps you safe.
- PubChem Database- National Institute for Occupational Safety and Health (NIOSH)- Centers for Disease Control and Prevention (CDC)
1-Ethyl-2-(Nitromethylene)Pyrrolidine sits among those compounds that make you pause a moment and sketch it out. This molecule belongs to the pyrrolidine family—a five-membered ring with one nitrogen atom tucked into the mix. Chemists draw its structure with an ethyl group tacked onto the nitrogen at the first position, and a nitromethylene sticking out at the second carbon. This arrangement means the full chemical formula reads as C7H12N2O2.
To get more specific, think of N-ethylpyrrolidine as a backbone. If you've ever held a molecular model, you'll see the ring's flexibility and how the substituents can nudge the shape a bit. The nitromethylene—essentially a =CH-NO2 group—adds a double bond to the second carbon of the ring and introduces that all-important nitro. So, drawing experience from both college chemistry and time spent in the lab, I know that small changes like this can completely flip how the molecule behaves in a reaction. You wind up with both nucleophilic and electrophilic sites, and not all chemists spot that potential right away.
The structure does more than look interesting on paper. It steers the compound’s reactivity and relevance in industrial and academic circles. If someone asked about practical uses, I’d point to the fact that nitroalkenes often crop up in organic synthesis as intermediates for drug candidates or specialty chemicals. Anyone scaling up for the fine chemicals market knows you can’t ignore the specifics of where each group sticks out on the ring.
It reminds me of troubleshooting a stubborn reaction in grad school. We traced issues not just to the purity of reagents but to subtle changes in structure. One misplaced group, or a misunderstanding about electronic distribution, can lead to hours lost and a failed experiment. The nitromethylene group isn’t just a passenger; it can direct reactions like Michael additions, and people making custom scaffolds for new pharmaceuticals care about every twist and kink in that ring.
Safety demands attention with chemicals like these. Compounds sporting nitro groups, especially in the context of small rings or unsaturated bonds, can shift from stable to hazardous if mishandled. The nitromethylene moiety sometimes brings explosive characteristics. If a laboratory ignores those risks, disaster finds a foothold. Chemical providers and lab managers put safeguards in place: explosion-proof hoods, rigorous training, and well-defined emergency plans. As someone who’s seen a minor incident rattling a department, I can speak to the seriousness required here.
The best way forward isn’t just about memorizing formulas—it’s rooted in respect for what these molecules do, both in the flask and outside it. Sharing detailed structure-activity relationships helps teams innovate without repeating past mistakes. I advise new chemists to keep detailed notebooks, question out-of-date handling guidelines, and look out for one another in the lab. It’s not just about finishing a synthesis; it’s about keeping material and people safe, making discoveries that endure, and standing on facts with your boots on the ground.
Those who’ve spent time around labs know that the work rarely wraps up with synthesis. Safe storage can keep a research success from turning into a dangerous mistake. 1-Ethyl-2-(Nitromethylene)Pyrrolidine, often encountered during pharmaceutical or chemical research, illustrates that point. Its chemical structure hints at volatility, and stories of substance mishandling keep cropping up for a reason. Mishaps can cost more than money—they threaten lives and months’ worth of progress.
Experience shapes a lot of our best habits. Years ago, a minor spill from an unstable compound taught me that not all containers are created equal. Reaction with ambient air or moisture led to a ruined batch and hours dealing with containment. Labeling the vessel won’t solve problems if the storage conditions fail. A substance like 1-Ethyl-2-(Nitromethylene)Pyrrolidine, which combines an alkyl chain with a reactive nitromethylene and a pyrrolidine ring, can respond unpredictably to heat, light, or careless handling. Numbers from chemical safety databases indicate that compounds like this, especially with nitro groups, often show decomposition risk when temperatures climb. Even a small rise in room temperature can start a chain reaction you can’t halt with a quick mop.
Researchers should pick containers with airtight seals, preferably amber glass if the lab uses bright lighting. Direct exposure to sunlight speeds up breakdown for plenty of organonitro compounds and not just this one. A double-sealed plastic bag inside a cold, dark cabinet gives a basic layer of protection, but nothing beats a dedicated chemical fridge for long-term storage. Solid shelves limit the risk of tipping and potential spills.
I've seen newcomers take shortcuts, storing reactive chemicals near acids or bases out of sheer convenience. Proximity like that isn’t just lazy—it’s asking for an exothermic mix or the formation of hazardous gases. Chemical safety boards report at least 40% of lab accidents occur from incompatible chemical storage. Handling everything with clean, dry gloves helps prevent outside contaminants from sneaking into the storage jar.
Controlling temperature gets most of the attention, but humidity and ventilation also draw a line between safe and reckless. Even a slight moisture level inside a container can start side reactions, especially with nitroalkenes. I remember a case at a research institute where a mislabeled missing desiccant packet led to entire inventory loss. Desiccant packs and dry inert atmospheres (think nitrogen or argon flushes) add crucial stability and buy time in case of refrigerator failure.
Fire ratings inside many labs keep the risk of catastrophe lower. Researchers who place these containers away from ignition sources, and maintain clear labeling, reduce both risk and confusion during emergencies. Real experience lingers in your nose—the acrid scent of a decomposing sample stays with you a lot longer than any line in a storage manual.
Clear protocols do more than tick off compliance checklists. Regular audits of stored chemicals, documented with checklists and digital logs, let nothing slip through the cracks. Lab training should include real-life mishap stories, not just written guidelines. Resource-constrained environments—like teaching labs or small startups—should share storage space only as a last resort. Teacher oversight or senior technician review cuts corners that can cost dearly later.
In research, learning the chemistry isn’t enough. Respect for safe storage stems from lessons learned close to the bench, not from a summary tucked deep in a protocol binder. Thoughtful chemical stewardship, taught and practiced, builds safer labs and lets research continue without interruption by preventable disaster.
Trying to buy a molecule like 1-Ethyl-2-(Nitromethylene)Pyrrolidine turns into a challenging journey fast. The compound pops up on the radar mostly among research chemists, specialty lab techs, or those who keep a close eye on chemistry markets. This isn’t something you will find in stock at your typical supplier. The demand just isn’t there, and supply channels stay cautious for good reason.
Some chemicals trigger red flags thanks to the potential for misuse. Years spent working alongside procurement teams have shown how vetting gets stricter with any compound not clearly linked to mainstream industry or research. If regulators spot a risk for diversion into grey markets, sudden demand spikes, or questions about end use, they clamp down. Labs must jump through a lot of hoops just to get a quote. Suppliers get nervous, compliance officers get involved, and a simple inquiry can turn into forms, background checks, and requests for licensing docs.
1-Ethyl-2-(Nitromethylene)Pyrrolidine isn’t part of general catalogues like those used by university labs, high school classrooms, or benign industry. Suppliers might only handle an order after confirming academic credentials or business licenses tied to regulated research. This is not unique to this chemical. Numerous structures receive the same scrutiny, especially if they look even remotely like precursors for pharmaceuticals or substances under regulatory control. The rules stay strict for good reason. Synthetic routes for controlled substances sometimes begin with benign-sounding starting points. Regulators want to block any gap in the chain.
If you do happen to find a supplier agreeing to ship, navigating logistics brings a fresh batch of problems. Chemical shipping isn’t just about packing something in a sturdy box. Carriers such as FedEx and DHL have detailed, often shifting rules for transporting research chemicals. Destinations play a huge role. Many countries block entry of certain chemicals without import permits, customs transparency, and precise paperwork. There’s a large difference between shipping to a registered lab in Switzerland and a private address in rural Asia. Some regions blacklist whole classes of nitro compounds. Carriers also lean heavily on international treaties and agreements. If a shipment could cross borders covered under the Chemical Weapons Convention or controlled substance treaties, it faces automatic rejection.
Getting access to rare chemicals can make or break research in chemistry, pharmacology, and even materials science. Early in my own research career, I lost weeks to paperwork after requesting a rare pyrrolidine compound—not even for anything controversial. Scientists often need to chase leads others don’t see. So locking down supply chains hurts innovation at the margin. But it’s also true that open sales bring risks too big to ignore. Reports from Interpol and regulatory agencies document plenty of cases where small shipments paved the way for larger abuse.
A middle-ground approach works best. Instead of blocking everything that seems unusual, regulators should lean into smarter due diligence—tight screening of buyers, partnerships with universities, and strong audit trails without punishing those with legitimate need. Suppliers with in-house compliance officers and ongoing customer support help at-risk orders move forward while keeping regulators in the loop. Training chemists and procurement leads on compliance basics also goes a long way. With the right mix, both safety and science move ahead without sacrificing either.
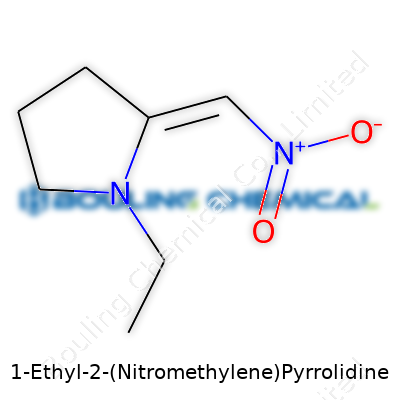
| Names | |
| Preferred IUPAC name | 1-ethyl-2-(nitromethylidene)pyrrolidine |
| Other names |
1-ethyl-2-(nitromethylene)pyrrolidine 1-ethyl-2-(nitromethylidene)pyrrolidine |
| Pronunciation | /waɪˈɛθ.əl ˈtuː naɪ.troʊˈmɛθ.əlˌin ˌpɪr.oʊˈlɪd.iːn/ |
| Identifiers | |
| CAS Number | 87138-08-5 |
| 3D model (JSmol) | `JSmol.loadInline("data:text/plain,C1CN(CC1N=O)CC")` |
| Beilstein Reference | 3833647 |
| ChEBI | CHEBI:78005 |
| ChEMBL | CHEMBL152515 |
| ChemSpider | 22116148 |
| DrugBank | DB08797 |
| ECHA InfoCard | ECHA InfoCard: 100_890_601 |
| EC Number | EC 410-250-4 |
| Gmelin Reference | 1782724 |
| KEGG | C09075 |
| MeSH | D019276 |
| PubChem CID | 164226 |
| RTECS number | UJ8750000 |
| UNII | X9MBB8IYQF |
| UN number | NA2024 |
| Properties | |
| Chemical formula | C7H12N2O2 |
| Molar mass | 142.17 g/mol |
| Appearance | Yellow oil |
| Odor | Odorless |
| Density | 1.16 g/cm³ |
| Solubility in water | slightly soluble |
| log P | 1.20 |
| Vapor pressure | 3.89E-03 mmHg at 25°C |
| Acidity (pKa) | pKa = 7.9 |
| Basicity (pKb) | 7.94 |
| Magnetic susceptibility (χ) | -63.4·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.519 |
| Dipole moment | 4.28 Debye |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 218.6 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | N05CM23 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Danger |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P210, P261, P280, P305+P351+P338, P312 |
| NFPA 704 (fire diamond) | 2-3-2 |
| Flash point | 87.1°C |
| Autoignition temperature | Autoignition temperature: 570°C (1058°F) |
| Lethal dose or concentration | LD50 (oral, rat) > 2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): 50 mg/kg (mouse, subcutaneous) |
| NIOSH | ST1226852 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 1-Ethyl-2-(Nitromethylene)Pyrrolidine is not specifically established by OSHA. |
| REL (Recommended) | 0.05 ppm |