Looking back at the push for heterocyclic chemistry in the mid-20th century, 1-ethyl-1H-pyrrole wasn’t grabbing headlines or driving petroleum moguls to build factories overnight. It waited on the sidelines while its parent, pyrrole, won attention from dye-makers and drug developers. As research turned to fine-tuning molecular scaffolds for pharmaceuticals and advanced materials, attention slid over to alkylated pyrroles—the simplest one being 1-ethyl-1H-pyrrole. During the surge of organic chemistry in the 1970s and 80s, as folks sifted through possibilities for tire vulcanization chemicals, solvents, and even OLEDs, chemists began to see promise in subtle functional group swaps on pyrrole. This is how 1-ethyl-1H-pyrrole began collecting citations in journals and popping up in conference talks, always as a sidekick to bigger, bolder molecules.
In labs and industrial settings, 1-ethyl-1H-pyrrole shows up as a clear, sometimes pale yellow liquid. It carries a sharp, amine-like smell with a hint of the briny scent you get in wet cellars—not as acrid as pure pyrrole, but unmistakably pungent. With its five-membered nitrogen ring and an ethyl group tacked onto the nitrogen, this molecule offers just enough reactivity to be useful without becoming too unstable for safe handling. Lab techs looking for a good intermediate for more complex synthesis often keep a bottle around, since it dissolves well in organic solvents and isn’t crazy expensive or prohibitively toxic.
The boiling point of 1-ethyl-1H-pyrrole sits around 140–150 °C at atmospheric pressure, making it possible to distill without much hassle. Density hovers near 0.93 g/mL, close to that of water but more slippery in your hands. It doesn’t mix with water, so try pouring it in a beaker and you’ll watch it float and spread like a thin oil slick. The structure carries a lone pair on the nitrogen, which takes part in hydrogen bonding, but the ethyl group moderates reactivity so it doesn’t just snap into reactions like more activated heterocycles. In practice, you can expect moderate stability in air but exposure to strong acids or halogenating agents will break it down quickly.
Suppliers label commercial bottles with essential data: 98-99% purity, CAS number 2719-96-6, batch number, and standard flashpoint warnings. Labels lay out the volatility hazard clearly, and any shipment above a few liters packs UN identification for flammable liquids. Buyers rarely see technical jargons like “suitable for spectroscopy” outside research-grade packaging; most users settle for analytical grade, which covers most synthetic and process needs. Keep it in tightly sealed glass containers or specialty HDPE because it creeps through soft plastics over time.
Chemists prepare 1-ethyl-1H-pyrrole by N-alkylation of pyrrole, starting with pyrrole itself and reacting it with ethyl bromide or ethyl iodide in the presence of a base—commonly sodium hydride or potassium carbonate. The mixture heats gently under nitrogen or argon to block air from oxidizing the ring. After a few hours, extraction and distillation follow. Experienced hands can boost yields with dry solvents and careful work-up, since any moisture encourages side products or opens the ring.
With the direct link between the nitrogen atom and the ethyl group, most reactions alter the carbon backbone or further decorate the ring system. Bromination or nitration gives access to 2- or 3-substituted derivatives, opening doors to new materials science possibilities, such as conductive oligomers for electronics or precursors for colorants. Electrophilic substitution dominates the reactivity, but under strong bases, that N-ethyl group can migrate or even come off in rare cases, making the molecule a curious starting point for structure–activity experiments in pharma labs or for growing novel types of organic semiconductors.
Ask around, and chemists might call it N-ethylpyrrole or 1-ethylpyrrole. Older literature sometimes lists it under more obscure headings, such as Ethylpyrrole-N1. For cataloging, the IUPAC name 1-ethyl-1H-pyrrole clears up confusion, and suppliers tend to stick with that to avoid mix-ups with isomers or related compounds. Tracking synonyms matters for customs, regulatory paperwork, and literature searches in patent filings.
No fun running an open bench with fumes wafting out, so anyone who works with 1-ethyl-1H-pyrrole meets it with gloves, safety glasses, and an active hood. Even a small spill can stink up a whole lab, so tight sealing and minimal headspace become rules. Flammable liquid rules apply: keep away from ignition sources, use grounding when decanting, and work in well-ventilated zones. Material safety data sheets spell out health risks—mostly irritation to skin and eyes, possible inhalation risks if vapor lingers, and warnings about long-term exposure, which studies still investigate. Getting disposal right means using certified incineration or solvent recovery rather than pouring down the drain.
With a structure that pioneers can tweak and test, 1-ethyl-1H-pyrrole finds its way into experimental drugs, specialty coatings, electro-optical devices, and probing intermediates in synthetic chemistry. Polymers that need stable, electron-rich building blocks lean on it for fine-tuning conductivity or light absorption. Some teams chase better organic solar cells, others want new anti-corrosive agents in paint, and pharmaceutical chemists sometimes try it as a stepping stone for central nervous system drugs. It’s never the only ingredient in these solutions, but standing in as a reliable node on the web of modern chemical tech.
The pace of R&D hinges on how easy it is to build new molecules from reliable starting points. Many groups use 1-ethyl-1H-pyrrole to model electron transfer, investigate deactivation patterns under light, or screen for interesting bioactive modifications. Researchers build new dyes with it, leveraging the subtle push-pull balance of the ethyl group to get wavelengths just right. I’ve read reports from university groups plugging it into long conjugated chains, seeking to squeeze out brighter emission in LED prototypes or next-gen imaging agents. Its reactivity attracts those exploring green chemistry, looking for less toxic routes and ways to recycle precious starting materials.
Toxicity data for 1-ethyl-1H-pyrrole, while less developed than for more common solvents, points to mild-to-moderate acute hazards depending on dose and exposure form. Animal studies show some irritation at high concentrations but not grim long-term organ effects. Still, researchers avoid direct contact and keep inhalation as low as possible, since persistent exposures to pyrrole derivatives can disrupt liver enzymes. Chronic risk is hard to nail down, since few people work with kilogram levels outside large-scale manufacturing, but the structure prompts caution given the family history—many five-membered nitrogen rings carry subtle risks, and regulators demand due diligence on waste streams and occupational exposure.
As industries shift toward smarter polymers, advanced composites, and flexible electronics, little pieces like 1-ethyl-1H-pyrrole step into more spotlights. Expect more patents leveraging its structure, more university groups chasing selective reactivity, and tighter safety protocols as the database on its effects grows. Not the molecule to launch a revolution overnight, but it won’t vanish from lab shelves any time soon. If chemists ever break through with more sustainable or high-performance materials, stepping stones like this one will have quietly laid the trail in the background.
Even outside the lab, a lot of us are affected by chemicals like 1-Ethyl-1H-Pyrrole, whether we know it or not. It’s a building block, not flashy on its own, but often the backbone of big projects in research and development. Its structure—an ethyl group attached to the five-membered pyrrole ring—makes it more than just another obscure chemical name tucked away in a catalog.
Researchers searching for better medicines or advanced smart materials turn to 1-Ethyl-1H-Pyrrole as a trusted starting point. It’s all about the chemistry: adding that ethyl group to the pyrrole ring changes reactivity and lets chemists steer reactions in ways that pure pyrrole can’t. In drug discovery, minor tweaks like this can make a difference between a compound that fails and one that ends up in clinical trials. The pharmaceutical world prizes these sorts of subtle changes for easing toxic side effects or improving how a medication travels through the body.
The material science side leans on compounds like this for making new polymers. Some of the most flexible plastics or conductive materials trace their origins to a pyrrole core. Substitute in an ethyl group, and researchers can shift the electrical performance or durability of the end product. Look at the ongoing search for cheaper, tougher, and more environmentally friendly electronics—this chemical finds a place on the workbench.
Art and science mix more than people think. 1-Ethyl-1H-Pyrrole shows up in the recipes for dyes and pigments, especially where subtle shifts in color matter. Pyrrole-based compounds get used in everything from vivid printing inks to artists’ paints that need resilience against sunlight and heat. That little ethyl group can introduce a tint shift or bring extra stability, helping colors last longer or stay truer. Reliable colorants count for more than just style—they tie into safety marks, signaling, and branding.
Synthetic chemists value small building blocks because they turn into more complex things. This chemical acts as a stepping stone—reacting with acids, aldehydes, or other functional groups to create molecules that don’t exist in nature. People may not realize how rarely a molecule gets used by itself. Most of the time, it’s about what that piece can become with the right tweaks.
A lot of these applications sound promising, but there’s more involved than dropping a chemical into a mix and walking away. Not all reactions play nice, and new chemicals can introduce safety problems or create waste that’s tough to treat. As someone who spent time in a university research lab, I’ve seen firsthand the balancing act: chasing innovation, staying alert for anything toxic, and always having a plan for disposal. The real world rarely delivers the neat, 100% yields shown in textbooks. Safety systems only stretch so far.
Shifting toward greener methods would help cut down risk and mess. More teams are building new processes—using fewer solvents, recycling byproducts, or designing lab setups with simpler cleanup. These steps not only protect workers and the environment but also cut costs, which makes adoptable solutions more likely in real-world manufacturing. Everyone wins—safer products, less pollution, and a smoother path for fresh ideas to make it from bench to business.
1-Ethyl-1H-Pyrrole might not be a household name, but it punches above its weight in chemistry circles. Whether it’s in pursuit of a brighter red or a breakthrough drug, this compound helps set the stage. Keeping an eye on safety, efficiency, and greener processes will shape how useful—and how sustainable—these applications remain in the long run.
1-Ethyl-1H-pyrrole grabs curiosity from chemistry students and researchers who enjoy exploring organic molecules. This compound forms when an ethyl group links itself to the nitrogen atom of a pyrrole ring. It’s oddly specific, but that’s the charm of organic chemistry — small swaps flip the way molecules behave. In the case of 1-ethyl substitution, the molecule has an ethyl group at the nitrogen position, unlike some more routine modifications at the carbon atoms.
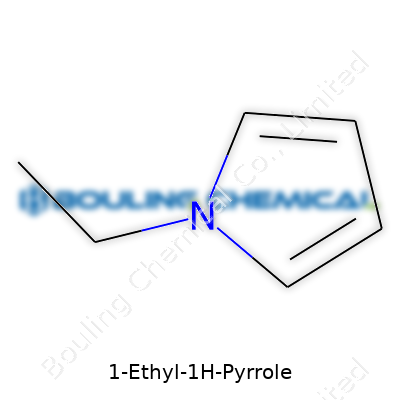
Breaking it down, the molecular formula sits at C6H9N. The backbone is pyrrole, a five-membered ring with four carbons and one nitrogen. Swapping one hydrogen on the nitrogen for an ethyl group (–C2H5) bumps the formula up to C6H9N from the parent pyrrole’s C4H5N. For visual thinkers, the structure shows a ring with alternating double bonds, a single nitrogen, and the ethyl chain sticking out from that nitrogen.
Putting an ethyl group on that ring changes everything in how this molecule greets the world. Personally, I struggled through my own organic chemistry labs to appreciate how much a tiny alkyl chain shifts reactivity and interaction with other molecules. Ask any medicinal chemist; swap a methyl for an ethyl, and suddenly you get a new therapeutic possibility — or a dead end. 1-Ethyl-1H-pyrrole doesn’t show up on pharmacy shelves, but its core, the pyrrole, appears in heme – the bit in hemoglobin hauling oxygen through your body.
Tweaks like this expose the hidden complexity at work. The ethyl group can impact how the molecule dissolves, how it interacts with other chemicals, and which enzymes chew it up. In environmental labs, folks test how different pyrrole derivatives survive or break down. Small changes ripple across the world, sometimes in ways you don’t spot until someone starts asking the right questions. Switching up a hydrogen for an ethyl group reveals chemistry’s sensitivity and creativity.
Getting the chemical structure right matters. I’ve seen classmates trip on molecular drawings, which drains time when running experiments or figuring out synthesis. If you map the structure wrong, you waste resources chasing something that never existed. Mistakes in chemical structure can creep into research papers, textbooks, and patents, tripping up progress. In the case of 1-ethyl-1H-pyrrole, clarity keeps research and experimentation on track. This underscores why there’s such need for chemistry educators to emphasize learning how to decode and draw these structures accurately.
Solving these kinds of problems in education and research benefits from plenty of hands-on drawing and software that checks structures. ChemDraw or open-source tools like Avogadro can cut down on the confusion. Teachers in underfunded schools or busy labs can make a difference by encouraging students to model molecules out of physical kits or online 3D environments. International collaboration and open publication of accurate molecular data can help, too. Reliable databases, easy access to structures, and peer-reviewed sources ensure fewer mix-ups as research keeps rolling.
These molecules aren’t just dry academic puzzles. Pyrrole rings hide in nature and industry, shaping medicines, pigments, and electronic materials. The more we dig into their modifications, the clearer it gets that understanding even modest molecules opens doors to unexpected discoveries. Every chemical tweak has consequences, both in the lab and out in the wider world.
Walk into any lab that deals with chemicals like 1-Ethyl-1H-Pyrrole, and the first thing you'll notice is clear organization. People who work around pyrroles know these guys aren’t milk or orange juice—you can’t leave them just anywhere. 1-Ethyl-1H-Pyrrole is a liquid with a flammable streak a mile wide, and it doesn’t play well with oxygen. So tossing it on a random shelf isn’t just lazy, it’s risky. I’ve seen what mistakes look like: a broken seal here, a bit of spilled liquid there, and suddenly the whole place smells strange, with everyone cursing under their breath.
The smart move with this chemical starts with a tightly sealed amber glass bottle. Light can turn pyrroles funny pretty fast, and the best way to keep things from turning weird is by blocking it out. Forget plastic bottles—those can let air through or react over time. I’ve watched people try to cut corners here, and every time something warps, leaks, or smells, there’s paperwork and headaches to deal with.
For the record, storage should mean cool and dry. Not freezer-cold—keep it out of the cold box that cakes chemicals in frost—but not anywhere near the heat of radiators, sunlight, or a spot that feels stuffy. Temperatures around the lower limit of room temp get the job done. Humidity is another sneaky problem. Moist air will get through a loose cap, and what you end up with isn’t pure anymore. You’ll see the color change, sometimes a bit of cloudiness, sometimes not much of anything except bad results later on.
The trouble isn’t just with ruined product. 1-Ethyl-1H-Pyrrole will burn quick if an open flame or spark is anywhere close. Vapors hang out in the air, and if there’s an old fan running, or careless use of matches, it takes one slip for a disaster. You also don’t want to sniff or touch it. Inhaling even a bit can give you a headache or worse—it’s a volatile compound for a reason, and skin absorption isn’t out of the question. Gloves and goggles seem like overkill until you’ve had a splash on your skin. Trust me, it’s not an experience worth repeating.
Keeping a label on the bottle isn’t just for the regulators. Always knowing what’s in that container avoids those “uh-oh” moments that end with someone grabbing the wrong thing. In the labs I’ve worked, a routine forms: use it, wipe the neck, screw the cap, place it back into a ventilated flammables cabinet away from oxidizers and acids. If anything spills or splashes, it gets cleaned immediately with the right absorbent—a soak-up rag will just spread it and stink up the place.
If shelf life matters, track the date when you opened the bottle. Don’t keep old stock sitting around. The chemical can slowly pick up water or turn, and someone unaware of its age will end up frustrated with unreliable results. Rotating stock keeps surprises out of your work.
No one wants a safety lecture, but accidents don’t give warnings. Simple habits—a cool spot, dry air, careful labeling, and limiting light—cut down on losses and danger. Using fresh gloves and keeping your face out of the fumes is part of keeping your day easy. In the chemical world, doing things the right way saves your work and keeps people safe. That’s just common sense.
1-Ethyl-1H-Pyrrole crops up most often in labs rather than under the kitchen sink. As an organic solvent or intermediate, its exposure risk stays pretty limited to chemists, students, and people running specialized manufacturing. This stuff carries a distinct smell, often described as a sharp or irritating whiff, which alerts the nose before the eyes spot a leak. I once caught a whiff of pyrrole during a college lab demo, and right away, my instinct was to step back and leave the beakers for someone else to handle. That instinct matters, because the real hazards go way beyond just an unpleasant smell.
The science points to several hazards. 1-Ethyl-1H-Pyrrole can irritate skin, eyes, and the respiratory system. Breathing the vapors can leave you coughing or short of breath. There’s little joy in that. Lab tests show this compound seeps easily into skin because of its small molecular size. Repeated or prolonged exposure pushes the risk further, possibly leading to headaches, dizziness, or more serious central nervous system symptoms. It’s not as notorious as benzene or toluene, but it doesn’t take a big spill for problems to start.
Anyone who spends time around organic solvents gets used to rules—goggles, gloves, open windows, and fume hoods humming. With 1-Ethyl-1H-Pyrrole, old habits pay off. Splash some on your skin, and it tingles or burns. Contact with eyes can mean pain and redness for days. Accidentally breathing vapor over a long afternoon ramps up the risk of headaches and nausea. No shortcut beats proper lab hygiene here. I’ve seen coworkers shrug off a few drops on the wrist, but the ones who rinse, report, and change gloves early end up in way less trouble.
There isn’t a massive stack of government toxicity reports about this specific pyrrole. Still, studies put structurally similar pyrroles in the “keep away from kids and pets” category. Breakdowns in water and soil don’t always come fast, and accidental release doesn’t magically vanish into the ground. This means workers must strictly contain and dispose of waste. Pouring it down the drain invites trouble that outlives the lab session.
Gloves, goggles, and fume hoods sound simple, but enforcement often slips with time. Clear labeling and regular safety reviews help. Some labs go so far as to schedule weekly cleanup inspections, with quick quizzes on the side just to keep everyone aware. I learned my biggest safety lesson from a clogged hood fan—air quality nosedived fast, teaching the crew to always test exhaust before starting. Swapping out solvents for less toxic ones remains the gold standard—nothing trumps avoiding hazardous chemicals altogether when possible.
One last point lands on reporting. If anyone feels off after using 1-Ethyl-1H-Pyrrole, they should speak up, not tough it out. Companies and schools benefit from tracking even mild symptoms; patterns often flag issues before they escalate. That transparency encourages both good science and safer workplaces.
Running a lab or a production line teaches you pretty quickly that purity figures aren’t just technical jargon. They’re the difference between a clean result and an experiment repeating itself for the wrong reasons. For 1-ethyl-1H-pyrrole, people expect a purity level of at least 97%. Higher-purity lots help chemists steer clear of unwanted side reactions when developing pharmaceuticals or new organic materials. It takes just a few stray contaminants to throw a wrench in those plans, so the push for 98% or even 99% purity isn't about marketing — it’s about necessity.
Some labs might relax this demand if they’re working on less complex reactions, but most stick to the highest grade available for consistency’s sake. Documentation from specialty suppliers backs this up; they’ll often publish GC-MS or NMR analysis straight on the product page, and the more detail, the more trustworthy.
Anybody who’s spilled a precious chemical understands why packaging choices deserve more attention. You’ll usually spot 1-ethyl-1H-pyrrole packaged in glass bottles, amber-tinted to shield it from stray light that can mess with its stability. I remember once working with a supplier who only offered this compound in 1-gram and 5-gram glass bottles, and it felt limiting — not everybody runs reactions on the same scale.
Some suppliers do offer options beyond glass: sealed ampoules crop up if shelf life or moisture worries top the list. Sometimes plastic-coated glass gets used for extra protection against breakage while moving chemicals between benches or shipping long distances. And believe it, packaging matters for more than just the convenience factor. Low-grade plastics can leach impurities or let small amounts of air slip in, leading to slow decomposition or shifts in color, especially for aromatic heterocycles like 1-ethyl-1H-pyrrole. It’s no fun draining your research budget because a reagent went off two months early.
Even with good suppliers, mistakes happen. Labs sometimes end up with bottles that have been sitting too long, and it doesn’t take much exposure for trace water or oxygen to creep in. This can seriously complicate reaction outcomes; I’ve seen yields drop off by half, thanks to contamination in starting materials. One way to fight back: always insist on the latest lot analysis and avoid suppliers who cut corners on documentation.
Bulk users could benefit from more customizable packaging sizes or on-demand bottling services, which reduce leftover “heel” stock that sits in storage for years. Small startups especially get squeezed here, since buying 100 grams of a compound for a project that will only use 10 grams means wasting money and risking degradation over time. Working with suppliers who understand these issues can help teams scale chemistry safely and cost-effectively.
Markets for niche chemicals like 1-ethyl-1H-pyrrole thrive on information sharing. Suppliers who offer live chat with technical experts or real-time purity certifications help buyers make informed choices. Sharing stories about frustrating experiences with poor packaging or out-of-date stock encourages companies to raise the bar across the board. Every field, from pharma to special polymers, benefits when chemical supply chains take end-users seriously.
Keeping focus on quality and giving room for flexible packaging means less waste, sharper data, and—most importantly—fewer headaches for the people doing the actual science.
| Names | |
| Preferred IUPAC name | 1-ethyl-1H-pyrrole |
| Other names |
1-Ethylpyrrole N-Ethylpyrrole |
| Pronunciation | /ˈwʌn ˈiːθɪl wʌn eɪtʃ pɪˈroʊl/ |
| Identifiers | |
| CAS Number | 766-99-4 |
| Beilstein Reference | 120924 |
| ChEBI | CHEBI:51641 |
| ChEMBL | CHEMBL16232 |
| ChemSpider | 12834 |
| DrugBank | DB08336 |
| ECHA InfoCard | 03e3b8eb-5387-4e74-abc2-3bcb0ca4a6b1 |
| EC Number | 610-116-5 |
| Gmelin Reference | 136228 |
| KEGG | C06581 |
| MeSH | D017910 |
| PubChem CID | 13529 |
| RTECS number | UE4207000 |
| UNII | TG8EWH9U49 |
| UN number | UN3436 |
| Properties | |
| Chemical formula | C6H9N |
| Molar mass | 109.16 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | pleasant, sweet, amine-like |
| Density | 0.891 g/mL |
| Solubility in water | insoluble |
| log P | 1.79 |
| Vapor pressure | 1.8 mmHg (at 25 °C) |
| Acidity (pKa) | 19.6 |
| Basicity (pKb) | pKb = 13.86 |
| Magnetic susceptibility (χ) | -40.2×10^-6 cm³/mol |
| Refractive index (nD) | 1.505 |
| Viscosity | 1.244 cP (25°C) |
| Dipole moment | 1.75 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 215.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | 122.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | –3259 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | N05CM21 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H226, H302, H312, H332 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P280, P303+P361+P353, P305+P351+P338, P370+P378 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 53 °C (127 °F) |
| Autoignition temperature | 215 °C |
| Explosive limits | 6–11% |
| Lethal dose or concentration | LD50 oral rat 670 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 > 5000 mg/kg |
| NIOSH | PY9675000 |
| PEL (Permissible) | Not established |
| Related compounds | |
| Related compounds |
Pyrrole 2-Methylpyrrole 1-Methyl-1H-pyrrole 1-Phenyl-1H-pyrrole 1-Propyl-1H-pyrrole 1-Butyl-1H-pyrrole |