Tuning into the past, 1-Cyclohexyl-1H-pyrrole-2,5-dione came into the spotlight as researchers searched for compounds with new bridgehead features and improved reactivity. Decades ago, scientists tried tweaking the classic succinimide structure with cyclohexyl substituents, hoping for benefits like better solubility and unique reaction profiles. Academic groups kept pressing, relying on fundamental organic synthesis knowledge and good, old-fashioned persistence. Lab notebooks from the ‘60s and ‘70s note the compound popping up in patent filings and medicinal chemistry trials. Even today, the skeleton still grabs the attention of synthetic chemists working to solve today’s challenges, blending old roots with fresh ideas in chemical innovation.
At its core, 1-Cyclohexyl-1H-pyrrole-2,5-dione stands as a small organic molecule that fuses the stability of succinimide with the bulk and hydrophobic nature of a cyclohexyl ring. Researchers tend to turn to this compound when they want to add rigidity or hydrophobicity to a molecular scaffold. The core gets picked up in experiments ranging from pharmaceutical intermediate studies to polymer work and organic electronics. Working with this compound, you start noticing how its rigid skeleton sometimes makes things easier in purification or boosts performance in certain chemical reactions.
Anyone handling it in the lab sees a crystalline solid, often off-white or pale in color, with a melting point hovering near 110-115°C. It carries a respectable molecular weight around 193.25 g/mol, which gets factored into calculations for stoichiometry and disposal. Dissolving in common organic solvents poses few hassles—ethyl acetate, chloroform, dichloromethane—these usually do the trick. That cyclohexyl ring tends to boost stability and lowers volatility compared to lighter, more fragile imides. Measurements from various laboratories show the compound keeps to itself in storage, provided you seal out excess moisture and light.
Most chemical suppliers stick to a standard purity level—rarely lower than 97%, often 99%—since impurities can ruin synthetic steps downstream. Bottles come labeled with proper hazard symbols, including warnings about possible irritation or environmental risks. Labeling usually includes batch number, production dates, and storage recommendations, which guide chemists tackling compliance and traceability requirements. On safety sheets, 1-Cyclohexyl-1H-pyrrole-2,5-dione falls under the category of non-carcinogenic, non-mutagenic, but it does not mean careless handling gets a free pass in the lab.
Preparing this compound still calls for some old-school synthetic chops. One routine path starts from cyclohexylamine, which reacts with maleic anhydride in a straightforward condensation. This method delivers the imide ring in a good yield, especially if you control the temperature and pH closely. Extraction and recrystallization polish up the crude material. Some labs swap in green chemistry tweaks, using solvents like ethanol or even mechanochemical setups to cut down on waste. Process improvements continue as chemists chase safety and cost goals, showing how a familiar compound can fit into new ethical frameworks.
Tinkering with 1-Cyclohexyl-1H-pyrrole-2,5-dione often means looking for ways to swap out substituents or open the imide ring. N-alkylation pops up as a go-to tactic, allowing connection to longer chains or bioactive scaffolds. Its imide core stays stable under mild conditions, but strong nucleophiles remind you of its hidden reactive side. Reductive ring opening, for example, leads to neat intermediates used in specialty plastics or drugs. Researchers also attach functional groups to the aromatic ring, broadening its utility for electronics or dye science. Every change brings a chance to tweak the properties and push into new territory.
Walking through catalogs, you’ll spot the same compound listed as N-Cyclohexylsuccinimide, N-Cyclohexyl-2,5-pyrrolidinedione, or Cyclohexylsuccinimide. The CAS number, 3886-69-9, helps clear up confusion during ordering and regulatory work. No matter which name gets used, the chemistry and risk profiles stay consistent, making paperwork more straightforward for seasoned staff tracking inventory or preparing import forms.
Keeping people safe takes more than skimming a safety sheet. Eye protection, gloves, and fume hoods matter, since the solid and its dust irritate skin and lungs. Spills get swept up with minimal fuss, but folks keep chemicals away from open drains. Fire hazards remain low for this compound, though some labs use flame-resistant lab coats as an extra precaution when heating larger quantities. Waste routes follow local hazardous chemical protocols, with efforts to neutralize any reactive residues before disposal. Auditors checking labs expect complete documentation for both use and disposal, which pushes chemical handlers to stay on their toes year-round.
This molecule pops up most often in pharmaceutical synthesis and specialty polymers. Scientists use it as a linker or intermediate when building more complex drug candidates, especially those hunting for improved metabolic stability. The electronics industry taps the compound’s planar core to craft advanced organic semiconductors, aiming for better charge transfer and tuneable band gaps. Even agricultural chemists eye it for slow-release formulations. Over the years, workers discover that the right tweaks unleash new uses, plugging the compound into experiments where both bulk and synthetic maneuverability matter. From experience, every inventive step with this molecule tends to inspire a dozen more ideas in unexpected fields.
University labs and commercial outfits keep running with this compound’s flexible core. Medicinal chemists explore it as a scaffold for enzyme inhibitors. Materials science teams build oligomers and composites using its robust ring system. Analytical chemists tweak its substituents to spin out reference standards. Environmental scientists track its persistence under sunlight and in soil. Every published article sparks a wave of lab meetings and brainstorming sessions, making it a touchstone for collaborative science. Real progress takes clear-eyed teamwork, patience with failed syntheses, and relentless curiosity about what variants might perform best under pressure.
People want answers when it comes to health and safety, so toxicologists run a full battery of tests. Animal studies show low acute toxicity, but caution stays high given how structural cousins can behave in odd ways inside biological systems. Skin contact sometimes brings mild irritation, while ingestion of high doses brings risks that echo other succinimide derivatives. Long-term data remains thin. Plant and aquatic toxicity gets similar treatment, since environmental chemists have learned the hard way that even small leachages cause headaches downstream. Keeping dose and exposure low remains the safest bet until more thorough research prints new guidelines.
Chemists never stop dreaming. The next decades look bright for 1-Cyclohexyl-1H-pyrrole-2,5-dione derivatives, especially in fine chemicals and next-gen materials. Improved green synthesis methods stand ready to lower the footprint and cost, giving labs more ways to experiment responsibly. Medical research keeps sniffing out fresh uses for the skeleton in both diagnostics and therapeutics, and the push toward biodegradable polymers opens new doors for its use in sustainable packaging. I see cross-disciplinary teams blending chemical insight with digital design to unlock possibilities well beyond what the original inventors could imagine. The journey keeps rolling, powered by questions that always lead to better ideas.
Some chemicals end up everywhere you look, and 1-Cyclohexyl-1H-Pyrrole-2,5-Dione—better known among chemists as a type of cyclohexyl-substituted maleimide—falls right into that camp. Its structure means it’s got a knack for forming stable bonds and standing up to tough conditions. I’ve seen plenty of companies lean on its versatility, not just because of the molecular details but based on how it solves real manufacturing and research challenges.
The push for tougher, longer-lasting plastics and specialized coatings never seems to let up. This compound finds a home in industries working on polymers with better thermal and chemical resistance. Epoxy resins and high-performance adhesives often rely on cyclic imides, and this one, with its cyclohexyl ring, brings a flexibility and durability balance most off-the-shelf imides can’t match. I’ve noticed engineers look for these properties in electronics, automotive components, and specialty coatings—especially where parts need to shrug off heat, moisture, or rough chemicals.
Folks in electronics pay close attention to what goes into their circuit boards and chip packaging. A material like this helps create layers and adhesives that don’t crack under heat or ruin the delicate connections inside a smartphone or computer. Modern gadgets get slimmer and more complex every year, which demands better, more reliable dielectrics and adhesives. The cyclohexyl group in this compound adds that extra tier of stability without making processing a headache, reflecting what I’ve seen in R&D labs chasing higher efficiency and miniaturization.
Innovators in drug discovery often gravitate toward structures like this compound because drug-like properties start with chemical foundations. Its imide group lets scientists build promising new molecules that sometimes end up as medications, depending on how the rest of the formula stacks up. In agricultural science, smart chemists use maleimide structures to develop crop protection agents, where strong hydrogen bonding and unique shapes can keep weeds and pests at bay without harming other chemistry in the field. Early trials and library screenings in these fields harness compounds like this to get ahead of pests or disease resistance.
The way this chemical reacts with thiols puts it in the toolkit for surface treatments, as anyone working in biomaterials or anti-fouling technologies can confirm. Surfaces of medical devices, diagnostic chips, or even underwater infrastructure often need a custom molecule attached just so. This cyclohexyl maleimide lets researchers graft on molecules, so they can tailor surfaces for better performance or biocompatibility. The fact that big research suppliers keep offering it says a lot about ongoing demand for advanced surface mods.
Every chemical with industrial legs brings not just promise but some questions. Environment, safety, and regulatory compliance always need a seat at the table. Responsible handling, regular toxicity studies, and full transparency in the supply chain can help manufacturers and researchers benefit from these advanced building blocks while keeping lab workers, consumers, and the environment protected. The best practices—wearing proper gear, waste minimization, resting on greener synthesis routes—help future-proof the benefits that materials like 1-Cyclohexyl-1H-Pyrrole-2,5-Dione offer, ensuring they solve real-world problems without adding new ones.
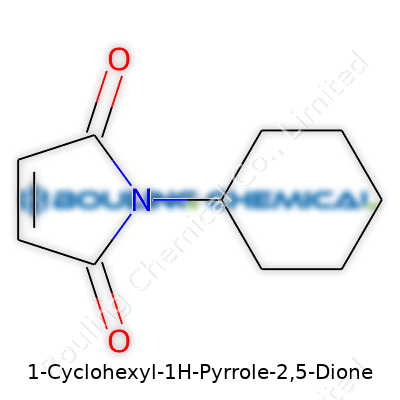
1-Cyclohexyl-1H-Pyrrole-2,5-Dione won’t win awards for the catchiest name, but its chemical story draws attention in labs for a reason. Picture a molecule with the formula C10H13NO2. It shows off a five-membered pyrrole ring, tagged with two carbonyl groups at positions 2 and 5—this instantly places it in the class known as maleimides. Tack on a cyclohexyl group connected at the nitrogen, and you’ve got a structure that hints at reactivity and utility, a mix that synthetic chemists keep reaching for.
In practice, this chemical sticks out because it combines the mildly reactive maleimide core—so valuable for linking molecules in bioconjugation or cross-linking reactions—with a bulky cyclohexyl group, which can shape how it fits or locks into other compounds. That level of control changes the way researchers build new polymers or design targeted drugs.
Chemists rarely look at molecules as only lines and circles on paper. Add a cyclohexyl group to a maleimide, and all sorts of changes ripple through its properties. In the lab, the cyclohexyl ring brings greater hydrophobicity compared to a simple hydrogen or methyl. I noticed this shift firsthand in aqueous reactions—usually, a cyclohexyl-maleimide separates itself from water, letting us play with phase separation during purification. That might seem minor, but in reality, it’s a practical edge in synthesizing peptides or making materials that need chemical precision.
People often overlook how such a molecule affects the world outside the lab. Polyimides that spring from maleimide chemistry form the backbone of aircraft insulation, flexible electronics, and components you won’t notice unless they fail. Researchers use 1-Cyclohexyl-1H-Pyrrole-2,5-Dione to tweak these polymers, switching up thermal stability or the ability to resist solvents—all because of a clever structural twist in one piece of the chain. A molecule like this becomes more than a classroom example; it defines the shelf life and performance of things we rely on without a second thought.
Every chemical, especially one as reactive as this, deserves respect. Maleimides draw in nucleophiles fast—great for quick bond formation but risky if not handled with the right safety gear. I recall hurried colleagues splashing too much in a glovebox, only to grip with sticky gloves as it cross-linked with whatever it touched. These lessons echo the guidelines set by professional societies and reinforce how basic safety can save time, money, and health. Early training focused so much on SDS sheets for a reason—familiarity with reactivity, not just memorizing formulas.
Waste is also an issue. Synthetic processes using maleimides generate organic solvent waste, sometimes contaminated with nitrogen or carbonyl-containing organics. Strategies like switching to greener solvents—water or ethanol replaces harsher options—or designing recoverable catalysts can shrink the footprint. In research projects, I pushed to optimize yields to cut down on leftovers. Little choices add up, making the difference between routine chemistry and sustainable practice.
Molecules like 1-Cyclohexyl-1H-Pyrrole-2,5-Dione push chemistry forward. By tuning their structures, researchers imagine better adhesives, smarter therapeutics, and more resilient materials. Rigorous science—and shared experience—help keep these advances meaningful, whether the molecule sits in a beaker or quietly holding together an engineered part halfway around the world.
I’ve spent enough time around labs and workshops to know that even the most routine chemical can cause big headaches if you get too comfortable. 1-Cyclohexyl-1H-Pyrrole-2,5-Dione stirs up particular concern because it sits in that space where skin, eyes, and lungs all demand protection. There’s history behind every rule on the safety sheet—ignoring them only buys trouble.
Some compounds play nice, but this one can irritate your eyes, skin, and airways. I remember spilling a related solvent on my glove—felt nothing at first, then watched as the glove started thinning from a slow chemical attack. The problem is not always instant pain; it’s often a slow burn or a sneaky vapor that gets inside your system. Inhalation can cause coughing and headaches. Skin contact? Redness or a rash. Let’s not even joke about what happens if it gets in your eyes. Working without goggles isn’t brave; it’s reckless.
Lab coats and gloves aren’t just for show. Nitrile gloves keep most organic solvents and this compound away from your skin. Too often, folks use latex gloves, which don’t hold up long. Chemical splash goggles beat out regular glasses every time, and a thick cotton lab coat blocks spills better than synthetics, which can melt and stick. Always check glove integrity—those pinholes spell disaster.
I once saw a colleague work with this class of chemicals outside the fume hood. Might as well breathe in irritation. Good lab practice puts all work inside a fume hood, not on an open bench. Ventilation eats the vapors before they reach your lungs. Don’t just trust in the window being open; real hoods make a difference, and the data backs it up—proper ventilation keeps exposure below the recommended limits.
You stash your food so it doesn’t spoil. Chemicals need the same respect. 1-Cyclohexyl-1H-Pyrrole-2,5-Dione likes a cool, dry, locked cabinet. Store away from acids, bases, and ignition sources. From experience, I’ve seen how a carelessly capped jar starts crusting around the edges—a sloppily sealed container lets in moisture and messes with purity, sometimes even causing pressure to build inside. That’s a recipe for a mess, or worse, a health emergency.
Splash or exposure always seems like someone else’s problem until you’re the one fumbling for an eyewash station. Know where the safety showers and eyewash stations are. Make sure that spill kits are stocked with absorbent materials appropriate for the chemical. Training beats panic—run drills often, because muscle memory shows up when nerves take over. Getting sloppy with disposal invites injury, too; harsh chemicals shouldn’t get washed down ordinary drains. Follow your community’s hazardous waste protocols—the environment isn’t immune to chemical mishandling.
Health isn’t just about avoiding pain today—it’s about protecting your future. Chronic exposure to irritants adds up in ways you don’t see in the moment. Review Safety Data Sheets before each batch or experiment. Open communication among teams helps everyone spot bad habits and fix them. Respect grows when everyone takes hazards seriously, and that’s how real safety culture is built. Every precaution becomes an investment in well-being, allowing work to continue without avoidable setbacks.
In any workplace or lab, the way chemicals get stored often decides whether things run smoothly or take a turn for the worse. Working with compounds like 1-Cyclohexyl-1H-Pyrrole-2,5-Dione, you only get one shot to get it right. This substance doesn’t catch the same spotlight as everyday household cleaners, but it deserves respect, no less. Mishandling can cause serious health risks or damage to property. Over time, I’ve seen what cutting corners leads to: unexpected fumes, ruined equipment, or even a scramble to evacuate a building. Prevention always beats emergency response.
Every chemist or technician will recognize this mantra: keep it cool, keep it dry, keep it away from sunlight. 1-Cyclohexyl-1H-Pyrrole-2,5-Dione reacts poorly with moisture and heat, which means storing it in a climate-controlled space. Most chemical storerooms have temperature monitors for a reason. The ideal spot hovers below normal room temperature, away from heaters or windows. Ultraviolet light can degrade certain compounds so nothing beats a dark, tightly sealed cupboard.
Don’t throw the jar on a shelf and walk away. Use containers with airtight seals—this reduces the chance of contamination and keeps hazardous vapors contained. Once, I saw a sloppy lid lead to an irritating odor that lingered for days. That kind of slip-up wastes product and invites trouble. Chemicals soak up moisture from the air, leading to clumps or unpredictable reactions, so keep desiccants around if humidity can’t be controlled.
Mixing chemicals never ends well unless it’s in a planned reaction. Store 1-Cyclohexyl-1H-Pyrrole-2,5-Dione far from acids, bases, strong oxidizers, and flammable goods. Clear, bold labeling keeps coworkers from making mistakes. A few times I've stepped into storerooms where faded, handwritten labels sat on similar bottles—asking for disaster. Use printed labels with chemical names, hazard info, and the date received or opened. Review and refresh them now and then.
Familiarity with the safety data sheet (SDS) isn’t optional. Everyone touching 1-Cyclohexyl-1H-Pyrrole-2,5-Dione should study those hazard statements. This compound can irritate eyes, skin, or lungs on contact or when inhaled. Wear gloves, goggles, and a proper lab coat, and make sure storage areas stay locked unless trained personnel can supervise access.
Spill kits and eyewash stations belong nearby. In a pinch, fast action limits harm, so nobody should hunt for safety gear in an emergency. Employee training on chemical handling and storage makes a workplace far safer—I’ve seen the difference between teams that rehearse spill response and those that wing it. The former handle emergencies calmly; the latter just hope to get lucky.
Responsibility covers more than just following policy. Double-check seals on containers, monitor storage temperatures through regular logs, and keep an eye on inventory dates. Store small stocks rather than buying in bulk. Set up routine safety audits so nothing slips through the cracks. In my experience, these daily habits add up. They prevent small issues from growing and help coworkers trust that someone’s keeping things under control.
Treating storage as an afterthought only invites headaches and danger. Careful planning, clear communication, and a good dose of caution keep risky compounds like 1-Cyclohexyl-1H-Pyrrole-2,5-Dione from threatening people or the workplace itself.
Anyone working in chemical synthesis, pharma labs, or materials research knows purity matters far more than it seems on paper. For 1-Cyclohexyl-1H-pyrrole-2,5-dione, suppliers usually offer purity levels starting from about 97%, with premium options going higher. Most researchers and industrial buyers don’t gamble with lower grades, since even a trace impurity can send a critical reaction sideways or degrade a coating. I’ve worked in a lab that accepted only 98% or better for anything hitting pilot batches. That extra percentage kept variables low and confidence high when results mattered.
Lab reports usually back purity claims through HPLC or NMR analyses. Some suppliers give certificates of analysis with every batch, and skipping that step can cause immediate headaches in regulated environments. For those in pharmaceuticals or electronic materials, the push is toward 98–99% to cut contamination risk.
Chemicals like this one don’t follow a one-size-fits-all model. Most research suppliers sell small packs, usually in 1-gram, 5-gram, and 25-gram bottles, often vacuum-sealed for stability. Academic teams pick these up for exploratory work or to test a reaction route, since ordering a full drum for twenty experiments makes little sense.
In industrial settings, the story flips. Bulk buyers ask for kilogram-scale bags or drums. Five kilograms, ten kilograms, and even twenty-five kilograms end up as standard requests. The business side decides based on throughput needs and available storage. In the coatings segment, for example, larger packs cut down on ordering frequency and lower shipping costs per gram. Bulk packaging must consider shelf life and avoid contamination, so most suppliers use sealed, moisture-proof containers and sometimes nitrogen blankets. Some facilities I've visited require double bagging and exclusion of certain plastics due to solvent sensitivity—a step that seems like overkill until a whole lot gets spoiled from packaging diffusion.
There’s a big difference in outcome between speculating on an unknown supplier and sourcing from a trusted name—clear labeling, quality paperwork, and verified handling often save projects from delays or rework. I’ve had colleagues run side-by-side tests using “technical grade” and “analytical grade” of compounds, only to see downstream processes break before the first phase ended.
Improper packaging can be just as dangerous as low purity. Moisture uptake in hygroscopic chemicals leads to variable concentrations by the time the jar gets opened for the tenth time. Those little things snowball in regulated fields with batch-trace requirements. One missed storage warning starts a chain where the finance side sees avoidable waste and production teams scramble to justify failed runs.
A smarter approach to chemical sourcing starts with asking for purity certificates for every order, preferably tailored to the specific lot. Auditing storage conditions and training staff to inspect packaging on receipt cuts the odds of ending up with degraded or off-spec material. For higher-risk applications, negotiating with suppliers to lock in batch traceability and validated packaging pays dividends. Direct relationships also foster accountability; I’ve seen suppliers provide immediate quality support on flagged lots when communication lines stay active. Production scale-up works out best when packaging and purity expectations align early instead of after problems spill over into production or product recalls.
Quality in chemicals like 1-Cyclohexyl-1H-pyrrole-2,5-dione doesn’t just come from the reaction flask. It flows from transparent sourcing to secure packaging—boosting reliability, compliance, and peace of mind for every user down the line.
| Names | |
| Preferred IUPAC name | 1-cyclohexyl-1H-pyrrole-2,5-dione |
| Other names |
N-Cyclohexylmaleimide 1-Cyclohexylmaleimide N-Cyclohexyl-2,5-pyrroledione |
| Pronunciation | /waɪˌsɪk.loʊˌhɛk.sɪlˌwʌn.eɪtʃ.pɜrˈoʊlˌtuː.faɪv.daɪˈoʊn/ |
| Identifiers | |
| CAS Number | 3886-69-9 |
| Beilstein Reference | 87820 |
| ChEBI | CHEBI:189450 |
| ChEMBL | CHEMBL138038 |
| ChemSpider | 177515 |
| DrugBank | DB07712 |
| ECHA InfoCard | 22-232-187-3 |
| EC Number | EC 221-741-1 |
| Gmelin Reference | 87772 |
| KEGG | C18333 |
| MeSH | D000900 |
| PubChem CID | 72561 |
| RTECS number | UF7700000 |
| UNII | F91GQ5MAR1 |
| UN number | 3077 |
| Properties | |
| Chemical formula | C10H11NO2 |
| Molar mass | 177.22 g/mol |
| Appearance | White to off-white solid |
| Odor | Odor: odorless |
| Density | 1.174 g/cm³ |
| Solubility in water | Insoluble |
| log P | 0.95 |
| Vapor pressure | 1.17E-4 mmHg at 25°C |
| Acidity (pKa) | 9.52 |
| Basicity (pKb) | 7.38 |
| Magnetic susceptibility (χ) | -65.2 · 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.567 |
| Viscosity | 1.207 mPa·s (25 °C) |
| Dipole moment | 3.69 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 389.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -317.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3324.2 kJ/mol |
| Pharmacology | |
| ATC code | N02BA01 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H317, H319 |
| Precautionary statements | P261, P264, P271, P272, P280, P302+P352, P333+P313, P362+P364, P501 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 113°C |
| Lethal dose or concentration | LD50 oral rat 4000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 1550 mg/kg |
| PEL (Permissible) | Not established |
| REL (Recommended) | 300 mg/kg |
| Related compounds | |
| Related compounds |
3-Methyl-1-cyclohexyl-1H-pyrrole-2,5-dione 1-Phenyl-1H-pyrrole-2,5-dione N-Cyclohexylmaleimide 1-Cyclohexylsuccinimide 1-Benzyl-1H-pyrrole-2,5-dione |