The roots of 1-Chloroformyl-4-Piperidinylpiperidine trace back to the surge in piperidine chemistry during the latter half of the 20th century. Back then, researchers hunted for novel compounds with potential across medicinal and materials science fields. The compound didn’t shoot to fame overnight. Early syntheses often suffered from low yields and tedious purification steps. Over the years, as organic synthesis improved, methods for accessing more complex piperidine derivatives, including this molecule, became possible. Some pioneering work came out of pharmaceutical labs exploring alternatives to well-known piperidine drugs, hoping these new molecules would show promise against emerging biological targets. Chemical supply firms began offering this compound for research, as the need for diverse building blocks in drug discovery exploded.
1-Chloroformyl-4-Piperidinylpiperidine stands apart with its two piperidine rings linked through a singular connection. The chloroformyl group gives it both reactivity and specificity, making it an intriguing intermediate. You find it mainly as a white to off-white crystalline solid, sometimes reported as a powder. Because of its structure, researchers don’t often encounter this molecule in day-to-day bench chemistry, but demand spikes in labs working in central nervous system (CNS) agent development or probing new chemical scaffolds.
This compound shows moderate solubility in polar organic solvents, which allows synthetic chemists to tune reaction conditions to suit their needs. Its melting point tends to fall in a moderate range, making it easy to handle at room temperature, without the volatility seen in lower-weight organics. The chloroformyl group lends distinct reactivity, and the presence of two basic nitrogen atoms gives it interesting acid/base behavior. Sensitivity to hydrolysis pushes labs to store it away from atmospheric moisture and light to maintain purity over time.
Suppliers list it under several catalogue numbers, specifying purity usually above 95%, but sometimes greater grades are available upon request. Labels state the chemical formula (C11H17ClN2O) and often display warnings due to the chloroformyl group's potential reactivity toward skin and mucosa. Safety data sheets accompany purchase, often including batch-specific analytical data: NMR, IR, GC-MS and sometimes even HPLC purity traces. Packaging ranges from amber glass bottles for small research quantities, fully sealed to prevent contamination and degradation.
Synthesis involves coupling piperidine precursors under controlled environments, often in anhydrous conditions. One uses a variant of the Vilsmeier–Haack formylation, tweaking reagents to place the chloroformyl group in the desired position. Catalysts, temps, and solvent choices significantly affect the outcome. These multi-step routes demand precision to avoid over-chlorination or by-product formation, meaning only experienced labs carry out the work at scale. Each step includes purification, often with liquid–liquid extraction, recrystallization, and column chromatography. Waste products, particularly organic chlorides, require careful neutralization and disposal, given environmental and safety regulations.
In synthetic chemistry, the chloroformyl arm opens up pathways for further transformation. Nucleophilic substitutions, acylations, and reductions take center stage. Researchers use this molecule to build more complex heterocycles or to append functional groups that tweak pharmacodynamic properties. Reductive transformations can convert the chloroformyl into corresponding alcohols or amines, expanding possibilities. In medicinal chemistry, these modifications allow for structure–activity relationship exploration, a staple for hit-to-lead drug development campaigns. Careful reaction set-up prevents unwanted side products, as the reactive group is sensitive to both nucleophilic and electrophilic reagents.
You might spot this compound listed under alternative nomenclature, depending on the supplier or research context. "1-(Chloroformyl)-4-piperidinylpiperidine" shows up in older catalogues, while some literature abbreviates it as "CFPP" for brevity. Trade names remain rare, but internal codes from specific pharmaceutical pipelines sometimes appear in patent literature. Checking cross-references becomes important, especially where regulatory filings or patent landscapes require exact, unambiguous identification.
Gloves, lab coats, and eye protection are non-negotiable. The chloroformyl functional group doesn’t just look nasty on paper; in real life, it irritates skin and membranes on contact. Fume hoods become a necessity rather than an afterthought. Emergency eyewash stations and spill kits must be at arm’s reach due to the potential for accidental exposure. For environmentally sound disposal, labs neutralize residues with base, followed by collection under hazardous waste protocols. Personnel training covers spill response, proper storage, and transport, reflecting not just legal requirements but hard-earned lessons from mishaps in the past.
The compound slots into medicinal chemistry platforms focusing on CNS-active compounds, pain therapeutics, and certain classes of antivirals. Large pharma turns to it for rapid assembly of potential drug candidates, as its structure fits well into privileged scaffolds known to interact with various neurotransmitter systems. Beyond pharma, researchers in agrochemicals or advanced materials dabble in piperidine derivatives when designing new products. Academic labs frequently pick it up for mechanistic studies that probe the behavior of reactive nitrogen compounds.
A good chunk of the literature using this molecule investigates its use as an intermediate, often in complex syntheses intended to yield biologically active entities. Teams racing to uncover the next blockbuster drug sometimes publish patents where close analogs to this compound form stepwise intermediates. Some computational chemists model binding affinities based on its core, seeking predictive insights before committing chemistry resources. A few teams also study its electron density distribution and reactivity patterns, unraveling how subtle tweaks to the molecule’s structure impact downstream reactivity and, by extension, biological activity.
While data remain scattered, researchers treat 1-Chloroformyl-4-Piperidinylpiperidine in the same vein as other piperidine-based materials that enter systemic circulation. Animal model studies often show that introduction of chloroformyl-containing rings can increase cytotoxicity, especially in cell lines with active transporters. Early studies revealed dose-dependent responses, with symptoms lining up with those from similar acylated amines: CNS depression, mild ataxia, and reversible hepatic impacts. Safety margins tend to be narrow, so best practice involves minimizing exposure during synthesis and purification. Regulatory bodies require toxicology screening before use in clinical or large-scale agrochemical pursuits.
The world of specialty chemical synthesis keeps broadening, and demand for advanced building blocks grows alongside. As researchers keep digging for more selective and potent agents, compounds like 1-Chloroformyl-4-Piperidinylpiperidine look set to stay relevant, if not essential, to early-stage discovery pipelines. Future tweaks may focus on improving synthetic yield, making the process greener, or modifying analogues to lower toxicity. One could imagine, with enough automation and AI-guided design, libraries of new molecules derived from this scaffold being handed off from computer to chemist in rapid succession. As regulatory focus tightens around process safety and environmental impact, work on cleaner, safer routes stands as both a responsibility and an opportunity for innovation.
In practical chemistry, purity isn’t just a number on a certificate; it can be the difference between something working reliably or blowing up in your face. Anybody who has spent late nights in a lab or fiddled with tricky syntheses in the real world knows that a small impurity can set off a domino effect, sometimes leading to failed reactions, garbage yields, or expensive troubleshooting.
1-Chloroformyl-4-piperidinylpiperidine finds its way into plenty of reaction schemes. Folks in pharmaceutical and research circles often hunt for this compound when preparing advanced intermediates, and what’s on the bottle label changes the outcome. Purity determines if you wind up isolating your shiny target compound or chasing ghost peaks that send process chemists down rabbit holes.
Most major suppliers list this material at a minimum of 97% purity. Bigger catalogs sometimes promise above 98%, but “analytical purity” means something different to every vendor. Some use GC, others use NMR or HPLC as the yardstick. There’s always the small print. I’ve seen “97% GC” turn out to hide water content or side products, and that number alone doesn’t always cover the question, “Is there anything in there that could ruin my reaction?”
Anyone dealing with tight regulatory standards or APIs knows a few percent of mystery material can spell disaster. For exclusive work in synthesis, 97–98% might cut it, especially if you're running pilot batches or process development. In scale-up or anything heading for animal work or the clinic, even bits of an unknown impurity will bring batch releases to a halt until it’s sorted out. So, checking the certificate of analysis, and looking for anything marked as “single impurity >0.5%” isn’t just bureaucracy—it protects your project and sometimes your staff.
Smaller labs sometimes have to settle for lots “as-is” from distributors, grabbing what’s on hand or what’s cheapest. In my own experience, cutting corners on source material rarely pays off. Sometimes, the knock-off is full of starting material or byproducts, so the cost saved on the initial purchase disappears during weeks of column chromatography or rerunning synthesis steps.
If you work with regulatory filings or fine chemical supply, the paperwork never ends. Certified reference materials and analytical validation become second nature when you’re trying not to lose an entire batch to a misjudged impurity. It gets even more important when customers expect to see a thirty-page dossier answering “What else is in there?” before placing an order.
Easy fixes don’t exist, but a few things help. Working with suppliers willing to show their full analytical runs, not just a reported percentage on a spec sheet, pays off in fewer headaches. Developing in-house techniques for confirming purity—like running comparative HPLC, NMR, or even LC-MS if it comes to that—offers peace of mind. Building relationships with suppliers who actually respond to questions makes a difference when you’re racing a deadline and need a replacement batch fast.
Anyone deep in process chemistry has had to purify “commercial grade” material themselves. It usually means biting the bullet and adding a column, distillation, or crystallization step before the chemistry even starts. It’s not glamorous work, but sometimes that’s how you save the whole batch from a disaster because some trace amine, solvent, or oxidized byproduct crashed your plan.
Bottom line: in the real world, the purity of 1-Chloroformyl-4-Piperidinylpiperidine usually comes quoted at 97–98%, but numbers on a spec sheet don’t tell the whole story. If your work depends on clean results—whether for R&D or regulated products—you learn to dig deeper, double-check data, and never take purity claims at face value.
Walk through any grocery store, peek at any medicine cabinet, and you’ll spot those little labels: “Store in a cool, dry place.” Over the years, it’s clear these words aren’t just filler. Product makers stamp them on packages for good reason. Ignore them, and you risk more than a dented box or a faded label. Somewhere along the line, I’ve learned that storage builds a bridge between a product’s arrival and its value in our daily life.
Take food for example. Leaving a chocolate bar in the glovebox on a summer day transforms it into a melted mess, streaked white with fat bloom and gritty on the tongue. Once I ruined a batch of spices by sticking them above the stove. Steam and heat leached away their punch, and what was left tasted about as lively as sawdust. Those small mistakes cost more than the price tag – they quietly chip away at trust in a brand, or the joy of cooking.
Medication slips easily into the danger zone, too. Life-saving prescriptions can lose strength if heat, moisture, or sunlight creep into the bottle. No one wants to find out that their asthma inhaler doesn’t work during an emergency. Medicines land in my own kitchen far away from the kettle and window, sometimes in a dedicated box below eye level. This tiny act protects something much bigger: our sense of safety.
Products face real enemies after they leave the factory. Excess heat speeds up chemical reactions, which turns fresh crackers stale and vitamins weaker. Dampness invites mold, clumping, or corrosion. Even oxygen seeps through packages, quietly breaking down oils and delicate flavors. Fluctuating temperatures do a number on creams, ointments, and electronics, causing separation, rust, or battery troubles.
Not many of us open a jar and study its label right away, but those “store below 25°C” reminders matter more than most realize. In my childhood, canned soup sat in the garage until a particularly cold winter. The cans bulged and popped when I opened them months later. One lesson stuck with me: storage isn’t just about tidiness, it’s about trust.
Practical steps make a difference. Cupboards away from the stove and oven tend to stay cool. Kept in airtight containers with a dry packet, snacks and pantry staples last longer. For valuables sensitive to heat or damp, like film or certain supplements, the fridge (with good labeling to avoid confusion) often keeps things stable. At work, I’ve used small hygrometers to check for excess moisture in storerooms. It’s not about high-tech solutions so much as steady habits.
Manufacturers could help by designing clearer labels. More than a dusty “cool and dry” note, maybe something as bold as “Keep under 25°C – your headache relief depends on it.” Retailers can make smarter choices with storage spaces, avoiding direct sunlight near windows. Refrigerated sections for sensitive items shouldn’t double as display for bottled water.
It’s tempting to shrug off storage directions as something that only matters to big warehouses or fussy cooks. Truth is, they touch all of us. By shaping our habits at home and work, we stretch value, safety, and satisfaction from every product we trust. Smart storage isn’t just about keeping things neat—it's about keeping life running just as it should.
Anyone who has ever browsed supplements, hemp products, or even protein powders has probably seen the letters “COA” pop up somewhere, maybe tucked away on a website or promised by customer service. The three letters stand for “Certificate of Analysis,” and businesses toss them around to reassure buyers. The question still lingers: do these certificates change anything in daily life, or are they just regulatory buzzwords?
I spent a few years working in a small quality control lab for a food manufacturer. Every shipment of ingredients landed in our receiving area with a hefty set of paperwork, and right at the top sat the COA. At first, I barely glanced at them, but after more than one surprise failed test, I learned to appreciate why a paper trail matters. A real COA lists which tests were run—think contaminants, heavy metals, microbial counts, sometimes batches for nutritional content. Most importantly, it gives both the actual results and the specific standards. It’s not just red tape; a solid COA lets you track whether what you hold in your hands actually matches what’s on the label.
During 2020, my local grocery saw a surge in new supplement brands. One elderberry extract labeled itself as “pharmaceutical grade.” A friend of mine, a pharmacist, bought a bottle out of curiosity and tried to find a COA. The company website did not post one, and emailed requests bounced. Eventually, after some digging, he found a typo-filled document with unclear origins. We ran a few at-home checks ourselves—like dissolving a tablet in water and tasting the bitterness—hardly scientific, but enough to know something was off. None of this would be necessary with reliable paperwork upfront.
Most everyday folks do not hunt for lab reports before grabbing vitamins at the store or online. Trust often sits with the brand, the packaging, or reviews. People sometimes assume all regulations are strict or enforced. The truth is that enforcement slips, especially for smaller producers or in fast-changing markets like CBD, herbal products, or even specialty foods imported from overseas. Fraud happens. Contaminants slip through. Only transparency keeps suppliers on their toes.
Instead of leaving the paperwork to gather dust, brands that value trust should post their COAs clearly, with easy instructions for finding batch numbers and reading the fine print. Customers can get familiar with what matters in these docs (lead, arsenic, allergens, pesticide residue, percentage of active ingredient) and ask questions before buying. Regulators could demand batch-level transparency for hot-button items—especially those sold for kids or with high safety risks. Some companies avoid the hassle because testing isn’t cheap. Focusing on short-term cost savings, though, only pushes problems into the future. One bad batch, one headline, and the whole brand can implode overnight.
I’ve seen good COAs save both companies and customers from headaches. Honest paperwork gives a layer of trust you can’t fake with flashy logos or influencer endorsements. In a crowded market full of promise, chasing after that basic document shows who actually stands behind their work—and who just hopes you don’t look too closely. The next time someone asks if a COA is available, they’re not nitpicking. They’re making sure they can believe what’s on the bottle. That’s a question worth asking.
Every chemical has a name, but in science, names get messy. Chemistry likes to double up, toss on prefixes, and pile on numbers that mean little to anybody outside the field. Here’s where the CAS number walks in—a no-nonsense way to sort out what’s actually what on lab benches or paperwork. For 1-Chloroformyl-4-Piperidinylpiperidine, that code is 77123-13-6.
Anyone who’s scrambled to order chemicals will tell you: a typo in a chemical name means a wasted day, sometimes a whole week. Plenty of compounds look similar but change one atom, and you’re no longer in the same game. The CAS number steps in as a quality-control checkpoint. If you punch in 77123-13-6, you skip confusion and land on the right compound, whether you’re getting it shipped from a lab supplier, checking a regulatory list, or browsing through a safety data sheet.
I’ve spent time sorting through catalogs where one molecule could have records under three different trade names. Sorting by the CAS number is the only way to loosen the knot. It saves time, money, and, once in a while, a professional reputation. This matters most when safety hangs in the balance—something many forget until a close call wakes everyone up.
This compound doesn’t show up in headlines, but it gets attention in certain circles—pharmaceutical research, for example. That’s where specific structures play a role in building bigger, more complicated molecules. Tracking and regulating these substances requires accuracy. If a chemical ends up mislabeled because somebody trusted a description and skipped the CAS number, things can spiral. Think of regulatory audits or supply chain disputes—problems multiply fast.
Even outside the lab, the unique CAS number ripples across safety, shipping, and storage. I remember a situation where two shipments, nearly identical in chemical name, landed on the same dock. One was strictly controlled, the other open for general use. Sorting by name alone would have ended badly. The CAS number stopped a mix-up that could’ve led to fines or worse.
Organizations can sidestep trouble with a few changes. Training everyone, especially those outside pure chemistry roles, about the power of CAS numbers stops mistakes at the source. Leaning into digital inventory tools helps as well. Systems can pull product info straight from the CAS registry and flag wrong entries before they reach the purchasing desk.
It’s tempting to focus only on the research, but every chemical is part of a bigger story: regulation, supply, safety, and policy all tug at these strings. Underestimating the importance of getting each CAS number right invites hassle for everyone. By locking in on 77123-13-6 for 1-Chloroformyl-4-Piperidinylpiperidine, people make sure that science, logistics, and common sense run together.
Anyone who depends on chemicals for work knows the clock starts as soon as you place the order. Every day shaved off a lead time can make the difference between meeting targets or fielding awkward calls about missed deadlines. It’s tempting to complain about delays, but moving dangerous or sensitive materials across highways, oceans, or through customs is more complicated than shipping a T-shirt from an online store. Behind each shipment, you have regulatory checks, temperature controls, and warehouse staff treating the goods with care.
Some customers have voiced their frustration about inconsistent timelines. I get it. A two-week quote that turns into a month can really throw off production schedules. The blame doesn’t fall only on manufacturers and distributors. Factors like port congestion, labor shortages, short supplies of specialty packaging, or strikes along the way throw off even the best plans. During the height of the pandemic, we saw delays stretch for months, and some specialty chemicals didn’t make it to buyers at all. It’s hard to explain to the boss why a crucial drum hasn’t arrived yet, especially when every update from the supplier just says, “We’re waiting for customs clearance.”
As someone who’s been caught in these logjams, I learned the hard way that asking detailed questions up front can save days of frustration. I don’t just ask for a delivery date—I ask whether the product ships from a local warehouse or an overseas plant. A chemical sourced domestically shows up fast, but imports face extra testing, paperwork, and border inspections. If the warehouse is across the state, freight usually rolls out in two or three business days. Shipments coming from India or China can linger at sea for weeks, then spend more days in customs.
Open lines help more than any automated portal ever could. My routine now involves checking in with both the supplier and the intended carrier. If they see paperwork issues early or catch a missing document, you get a chance to fix things before your order sits in a warehouse for another week. There’s real value in building relationships with your logistics contacts, so they give you straight talk instead of canned responses. Don’t just press “buy” and disappear—follow up and make your urgency clear.
Manufacturers and shippers who give clients a live timeline and prepare for roadblocks outshine the competition. Some set up status alerts that go out the moment cargo passes checkpoints. I’ve seen teams who assign staff just to check container updates and reach out with news or bad weather warnings. This isn’t always standard, but it helps set expectations and keeps everyone moving in the same direction.
If you’re always in a rush, finding a chemical supplier with regional stock or distribution partners nearby can cut wait times dramatically. Pre-negotiating blanket agreements or placing standing orders also gives both sides more flexibility and incentive to hustle. Cutting back on last-minute requests keeps surprises down and stress levels steady.
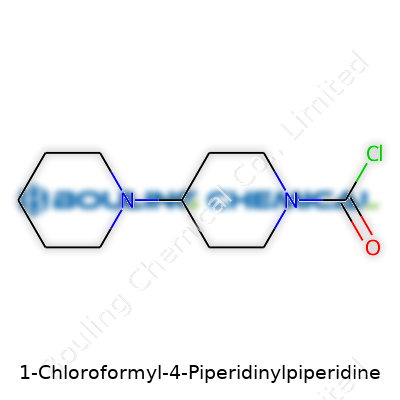
| Names | |
| Preferred IUPAC name | 1-(1-chlorocarbonylpiperidin-4-yl)piperidine |
| Other names |
1-(Chloroformyl)-4-(piperidin-1-yl)piperidine 4-(Piperidin-1-yl)piperidine-1-carbonyl chloride N-(4-Piperidinyl)piperidine-1-carbonyl chloride |
| Pronunciation | /waɪn-klɔːr.əˌfɔːr.mɪl-fɔːr-paɪ.pəˌrɪd.ɪn.ilˌpaɪ.pəˈrɪd.iːn/ |
| Identifiers | |
| CAS Number | [77145-89-4] |
| Beilstein Reference | 1463002 |
| ChEBI | CHEBI:51875 |
| ChEMBL | CHEMBL1257073 |
| ChemSpider | 120410 |
| DrugBank | DB08761 |
| ECHA InfoCard | ECHA InfoCard: 100.145.273 |
| Gmelin Reference | Gmelin 83628 |
| KEGG | C18368 |
| MeSH | D026521 |
| PubChem CID | 10217870 |
| RTECS number | GV8933000 |
| UNII | K8N0306A7U |
| UN number | UN3437 |
| Properties | |
| Chemical formula | C11H17ClN2O |
| Molar mass | 316.82 g/mol |
| Appearance | White solid |
| Odor | Odorless |
| Density | 1.19 g/cm3 |
| Solubility in water | slightly soluble |
| log P | 1.07 |
| Vapor pressure | 0.0012 mmHg at 25°C |
| Acidity (pKa) | 14.21 |
| Basicity (pKb) | 3.79 |
| Magnetic susceptibility (χ) | -81.0 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.578 |
| Dipole moment | 4.79 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 360.6 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | N05CD11 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes severe skin burns and eye damage, may cause respiratory irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS05,GHS06 |
| Signal word | Danger |
| Hazard statements | H302 + H312 + H332: Harmful if swallowed, in contact with skin or if inhaled. H315: Causes skin irritation. H319: Causes serious eye irritation. H335: May cause respiratory irritation. |
| Precautionary statements | P261, P280, P304+P340, P305+P351+P338, P405, P501 |
| NFPA 704 (fire diamond) | 2-3-0 Health:2 Flammability:3 Instability:0 |
| Lethal dose or concentration | LD50 oral rat 365 mg/kg |
| LD50 (median dose) | LD50 (median dose) of 1-Chloroformyl-4-Piperidinylpiperidine: **"LD50 oral (rat) = 142 mg/kg"** |
| NIOSH | VZ3150000 |
| PEL (Permissible) | Not Established |
| REL (Recommended) | 10 ppm (40 mg/m3) |
| Related compounds | |
| Related compounds |
1-Formyl-4-piperidinylpiperidine 1-Acetyl-4-piperidinylpiperidine 1-Benzoyl-4-piperidinylpiperidine 1-Chloroacetyl-4-piperidinylpiperidine 4-Piperidinylpiperidine N-Formylpiperidine N-Chloroformylpiperidine |