Stories of chemical innovation often track with industrial progress, and 1-Butylpyrrolidin-2-One is no exception. This compound started getting attention in the late 20th century as part of a search for better solvents for the pharmaceutical, polymer, and agricultural sectors. Early on, chemical engineers sought to improve on N-methylpyrrolidone (NMP), aiming for a substance with lower toxicity that didn’t compromise on performance. Efforts like these led researchers in Germany, Japan, and the United States to explore longer side-chain pyrrolidones, which brought 1-Butylpyrrolidin-2-One into focus. Investment in research at a time of growth for drug synthesis and new materials fueled both lab work and patent activity, pushing this compound from a pure research subject into concrete industrial application and trade.
1-Butylpyrrolidin-2-One stands out for its performance as a polar aprotic solvent. Chemists working on pharmaceuticals or specialty polymers prefer it for its ability to dissolve a wide range of organic and inorganic compounds. The product typically comes as a colorless to pale yellow liquid and, unlike many other solvents, offers a high degree of stability under both acidic and basic conditions. Its use stretches beyond the lab. Industries dealing with coatings, printing inks, and adhesives rely on its efficient solvency. Even crop protection products have benefited, because the solvent can boost delivery of active ingredients without adding significant toxicity or risk for the user.
The properties of 1-Butylpyrrolidin-2-One turn it into a favorite among industrial chemists. Its melting point hovers just below room temperature, while its boiling point nudges above 240°C, offering a broad liquid phase useful in both high- and low-temperature applications. Miscibility with water and most organic solvents grants real versatility when developing formulations. Thanks to its relatively low vapor pressure, losses due to evaporation drop significantly compared to other solvents. Its molecular stability under thermal stress lets it serve in demanding processing conditions, where many other solvents would degrade or introduce unwanted contaminants. The chemical features, shaped by both the heterocyclic ring and the butyl chain, support a rich set of reactions in organic synthesis, making it valuable far beyond routine solvent work.
Specifications tell buyers and users what to expect. High-purity 1-Butylpyrrolidin-2-One generally comes at >99% purity, with trace moisture dictated by the chosen grade; pharma-grade versions tend to cap water content well below 0.2%. Common technical sheets outline refractive index, density, retention time for chromatography, and UV absorbance at key wavelengths. Shipping containers, labeling, and paperwork all follow international codes set by REACH in the EU and TSCA in the US. Proper UN numbers and GHS pictograms appear on labels to highlight both physical and health risks. Selecting the right batch means sifting through this technical data, balancing the purity needs of end users with cost pressures that every operation faces.
Making 1-Butylpyrrolidin-2-One typically starts with gamma-butyrolactone. Chemists react this precursor with n-butylamine under controlled conditions. This ring-opening reaction forms the amide linkage at the “2-One” position, while the butyl group builds alkyl chain length, tuning properties compared to NMP. Fine-tuning reaction temperature, pressure, and solvent system all affect final yields and byproduct formation. Several companies have smoothed the process by using continuous-flow reactors, which help maintain higher product quality and minimize waste. Handling the process in sealed vessels cuts down on exposure to amines and prevents oxidation during upstream purification.
1-Butylpyrrolidin-2-One brings an adaptable backbone, letting chemists functionalize either the ring or the butyl chain. Electrophilic substitution at the nitrogen or oxygen in the pyrrolidinone ring opens up new derivative compounds. These have found routes into active pharmaceutical ingredients (APIs), specialty surfactants, and high-performance polymers. Hydrogenation at the ring or oxidation at the butyl chain grants access to subtle changes in solvent behavior or bioactivity, feeding both academic curiosity and industry demand. Its strong polarity, paired with moderate basicity, also lets it act as a mild catalyst or co-catalyst for select condensation reactions, widening its niche in green chemistry projects.
In catalogs and literature, this compound crops up as 1-Butyl-2-pyrrolidone, N-butylpyrrolidone, or abbreviated BPy. Sometimes, manufacturers put out proprietary blends using trade names, though the base chemical structure rarely changes. Checking synonyms helps ensure that users source the compound that delivers the physical and regulatory profile required, limiting unforeseen errors in development or scale-up.
Healthy skepticism follows any solvent, and BPy is no different. Acute toxicity sits below that of NMP, but prudent operators treat it with respect. Inhalation or skin contact can cause irritation, and long exposure still raises concern about reproductive toxicity. Handling calls for nitrile gloves, good ventilation, and splash protection around open tanks. Facilities shipping or storing drums need secondary containment and MSDS-tracked logs. Routine monitoring for air levels in production spaces squares with worker safety goals, while disposal routes lean toward licensed hazardous waste handling in line with local and international laws. Checking each delivery for purity and checking emission controls at the plant keep standards high and help head off fines or worker complaints.
Manufacturers in electronics, pharmaceuticals, agrochemicals, and specialty coatings find a slot for BPy. Formulation chemists value its strong solvency for epoxy and polyurethane resins. Drug makers push it into API salt or cocrystal formation steps, especially when water-sensitive intermediates are involved. Growth for lithium-ion battery binders created another demand spike, giving the electronics sector a solvent that works well without ramping up environmental risk. Farmers benefit from adjuvants or pesticide formulations where controlled release and improved leaf adhesion matter. Each of these applications reflects specific performance-driven needs, with BPy holding steady in competition with older, riskier solvents.
R&D chemists tackle limits imposed by regulatory bodies and market trends. Recent years brought shifts, as more labs chase greener chemistry approaches or need solvents not flagged by REACH or EPA watchdogs. BPy gets trialed as a substitute in NMP-forbidden markets, but projects don’t stop there. Research into BPy-composites aims for even safer, more robust alternatives, combining it with ionic liquids or biodegradable polymers. In synthesis, teams aim to lock in selective reactions or minimize byproduct formation with the help of BPy’s polarity and stability profile, reducing downstream purification burdens and trimming cycle times. Such efforts filter rapidly into tech transfer and intellectual property filings, keeping the field busy and competitive.
Toxicologists run animal studies, cell-line work, and environmental monitoring to clear up risk profiles. Acute oral or dermal toxicity falls into the moderate range, but chronic studies prompt more caution—lab rodents exposed over months show liver enzyme changes, shifting long-term LD50 and NOAEL values. Recent advances in in vitro systems and environmental fate modeling point to moderate persistence in soil and water; this presents a clean-up challenge if spills occur. Reviewing the literature, I notice a clear caution against disposal through routine drains or municipal waste streams, as aquatic toxicity for invertebrates still raises red flags. Each year brings more nuanced data as testing guidelines evolve, giving regulators plenty to work with as they update material safety datasheets and handling advice.
Industry momentum toward safer and greener chemistry looks set to keep BPy demand strong. Researchers watch for structural tweaks, such as adding hydrophilic moieties or switching to bio-based feedstocks, aiming to lower both carbon footprint and biological hazards. Battery tech and semiconductor fabrication may trigger more pilot plant trials, especially as solvent restrictions push older NMP-based blends out of favor. Regulatory shifts, both at home and abroad, require every player to keep pace—pushing some to invest in recycling streams, or to re-engineer process steps for solvent minimization. Trade groups, academic coalitions, and independent labs contribute data and insight, sharing advances in reactivity, recovery, and biodegradability. The next few years will likely see expanded use, but the pressure to innovate cleaner, safer, and more sustainable products won’t let up.
The name 1-Butylpyrrolidin-2-One might sound technical, but people in labs and manufacturing plants know it for its unique qualities. This compound, a close cousin of N-methylpyrrolidone (NMP), often finds a spot as a solvent in a variety of chemical processes. Tech companies looking for safe and efficient alternatives have started to pay more attention to it, especially as regulations for traditional solvents continue to tighten.
Electronics manufacturing runs smoother with the use of 1-Butylpyrrolidin-2-One in cleaning solutions. Its balance of strength and low odor allows circuit board makers to remove flux residues without too much hassle. Factories coat wires and devices using specialty paints and varnishes that need a solvent able to dissolve tough resins and polymers. This compound keeps the paint consistent and helps it spread evenly, avoiding bubbling or streaking as it dries.
In the world of plastics and advanced materials, 1-Butylpyrrolidin-2-One steps up as both a solvent and a reactant. Polymer chemists appreciate how it can break up chunky chemical mixtures, helping everything blend smoothly. It also makes processing easier for resins used in adhesives and laminates, giving end products better strength and flexibility. Consumer goods made with high-performance polymers often owe some of that adaptability to solvents like this one.
It doesn't stay only in the factory. Personal care products sometimes rely on 1-Butylpyrrolidin-2-One for its ability to dissolve fragrances and ingredients that resist mixing with water. Some hair dyes, nail polishes, and skincare formulas use it to carry active ingredients deep into the product, squeezing out more value per bottle. Companies keep an eye on the latest toxicology data to make sure they're not taking risks with people's skin, following guidance from bodies like the European Chemicals Agency.
Avoiding pollution and health risks has become a hot topic. Older solvents built up a bad reputation for harming workers and the environment. 1-Butylpyrrolidin-2-One brings a friendlier profile, breaking down more easily in nature and showing lower toxicity in early studies. Still, long-term research needs to stick with it — exposure limits and safe handling rules must keep pace with growing demand. Producers now supply it with clear guidance on workplace ventilation, spill control, and personal protection.
Researchers out there work to make green chemistry a bigger reality. While 1-Butylpyrrolidin-2-One already offers a safer choice than some solvents, nobody expects miracles from one molecule. Efforts continue to create even less harmful options made from renewable feedstocks. Companies share their findings on solvent performance and emissions to avoid old mistakes. Regulatory bodies watch closely, setting rules that push innovation without stalling progress.
Having worked in a materials lab, I've seen how a solvent like 1-Butylpyrrolidin-2-One quietly improves processes that touch daily life, from gadgets to makeup. It never grabs headlines, but its influence shows up every time somebody wants safer production and cleaner formulas. Bringing together industry standards, health data, and responsible sourcing will shape how companies keep making smart choices in this space.
Many people have never heard of 1-Butylpyrrolidin-2-One, yet this solvent has carved out a spot in labs and industry as a go-to for formulating coatings, inks, and cleaning agents. The catch is, scientists and workers deal with it up close, meaning vapor can get into the air, skin may touch it accidentally, or a spill might find its way onto a lab bench. Once, I watched a technician fumble a bottle of NMP—a similar compound—and everyonerealized how unguarded hands can become a route for absorption.
Rushing through cleanup or skipping gloves makes it easy for chemicals to get under the radar. Research shows 1-Butylpyrrolidin-2-One can irritate eyes and skin. People might develop redness, a burning feeling, or even a rash. Inhaling higher vapor levels stings the respiratory tract. Some are tempted to brush it off as “just another solvent,” but experts from the European Chemicals Agency list it as a substance with suspected reproductive toxicity. This isn’t just a dry fact; mistakes here can threaten real families planning a future.
Peer-reviewed studies pop up in journals on chemical safety, pointing to health effects when exposure runs too high or too long. Rodent studies flagged up changes to the liver and kidneys after repeated doses. In some experiments, embryos from exposed animals didn’t always develop as they should have. While most adults in the workplace won’t face repeated exposure at those levels, a lackadaisical approach opens the door to problems over time.
Trust gets built by owning up to risk and routine. From what I've seen on factory floors and in universities, compliance with safety data sheets separates safe sites from dangerous ones. In the US, the EPA and OSHA both recommend glove and goggle use, and they emphasize the point: just because something is “approved for industrial use” doesn’t mean it’s without hazards. Regulations in Europe and Asia keep tightening, not just for the environment but for people, too. These aren’t just paper rules—they reflect tragedies from lax oversight.
A locked cabinet, spill kit ready to go, and clear labels make a huge impact. Some colleagues roll their eyes, but those simple steps limit harm. Regular training gets people talking about near-misses and close calls. Leaders have to demonstrate that reporting a leak counts as courage, not complaining. Many of us undervalue proper storage, forgetting that even a small leak can build up vapor in a closed space.
1-Butylpyrrolidin-2-One closely resembles NMP, another solvent drawing attention for its risks to reproductive health. A 2022 review in Chemical Research in Toxicology stressed not just acute harm but long-term risk of repeat exposure—something many users shrug off until medical issues arise. As regulations change, producers may substitute one chemical for another, but that swap can shuffle problems, not erase them.
Nothing beats awareness. PPE—those gloves, face shields, and proper lab coats—never go out of style. Ventilation systems deserve the same attention as complex instruments. Room designs, workflows building in rinsing and waste management, and honest conversations about close calls bring down exposure. I’ve learned mistakes are shared fastest in places where management listens instead of punishing. That culture matters as much as equipment.
Storing chemicals isn’t one of those things people get excited about. But I’ve seen what happens when folks treat storage like an afterthought. 1-Butylpyrrolidin-2-One, often found in labs and some manufacturing facilities, deserves real attention. This solvent doesn’t shout danger at first glance, but a little thought goes a long way.
Heat, sunlight, and humidity turn stable solutions into a chemistry set you never wanted. This compound asks for storage in a cool, dry spot—think 15 to 25°C, away from pretty much any heat source or sunny patch. Most lab fridges and climate-controlled rooms fit this range easily. Moisture inside the bottle can nudge it towards decomposition, and wet storage areas welcome bad surprises.
Oxygen sometimes acts like a slow but determined enemy for chemicals. Sealed containers, preferably with nitrogen or another inert atmosphere, cut that risk down. UV light may not snap this solvent’s structure in two overnight, but stray light over a few weeks builds up trouble. Opaque or amber containers extend the shelf life and reduce headaches.
Some labs use containers made out of several types of plastic, glass, or even metal. I always stick to tightly closed amber glass bottles for 1-Butylpyrrolidin-2-One. The chemical won’t eat through glass, and glass doesn’t interact back. Hard plastics rated for solvents suit short-term use, but glass goes the distance. Lids need a solid seal: no loose caps or temporary covers.
Chemical storage disasters don’t just happen in Hollywood. Keeping this compound away from strong acids, ably-bases, and oxidizers cuts the risk of unwanted chemical reactions. I use separate shelves, labeled clearly, far from anything that could kick off an exothermic mess. Store away from open flames and direct heat—a solid rule for nearly everything flammable or volatile. Safety Data Sheets back up the need for segregation; cross-contamination haunts folks who try to save space.
One time I found an unmarked solvent bottle on a back shelf. Turns out it had been there for years, label long faded. The uncertainty it caused cost more time and resources than proper labeling ever would have. Each container should show the chemical name, concentration, date received, and hazards. It’s basic, but it keeps everyone honest and safe. Electronic tracking helps for bigger operations, making audits less painful.
Vapors from solvents sometimes sneak up on you, especially if there’s a slow leak. A storage area with strong ventilation—think fume hoods or designated vented cabinets—slows vapor buildup, protecting air quality. Spill kits near the storage area prevent a scramble when something tips or springs a leak. Absorbent pads, neutralizers, and proper personal protective equipment all help.
People shape safety. Written policies alone can’t prevent mistakes. I’ve seen smart folks gloss over the basics, thinking a few shortcuts won’t matter. Regular training makes safe storage second nature: where to put solvents, how to check for leaks, what to do during a spill. Peer reminders stick better than posters in the break room.
Storing 1-Butylpyrrolidin-2-One safely means no guesswork and fewer surprises. The rules look simple—a cool, dry place, away from light and incompatible chemicals, with careful labeling and ventilation. But people following those habits, year in and year out, make the biggest difference. The result: fewer accidents, fewer wasted materials, and peace of mind for anyone working nearby.
Looking at 1-Butylpyrrolidin-2-one, you find eight carbons, fifteen hydrogens, one nitrogen, and one oxygen woven together. The molecule—the name already hints—combines a five-membered lactam ring called pyrrolidinone with a straight butyl group stuck to the nitrogen atom. Picture it: the pyrrolidinone forms the core, and the butyl chain hangs off, almost like an arm raised in a crowd.
1-Butylpyrrolidin-2-one features a five-membered ring that includes four carbons and a nitrogen. There’s a double-bonded oxygen attached to one carbon in the ring, capturing the lactam vibe. From the nitrogen, a four-carbon butyl side chain reaches out. Chemically, that formula, C8H15NO, sets this compound apart: the backbone lets it blend with both water and nonpolar substances. That makes it powerful as a solvent, especially where stubborn residues resist washing out.
Solvents are part of daily life, whether unclogging engines or cleaning up after an oil spill. 1-Butylpyrrolidin-2-one excels at dissolving a wide mix of materials. That versatility owes plenty to its chemical structure—the butyl group brings enough bulk and flexibility to slip between molecules; the ring anchors the polarity. It doesn’t just work as a neat laboratory curiosity; manufacturers reach for it in sectors as broad as textiles and electronics.
It’s reassuring to know its safety record is much more favorable than some traditional choices. Facts show lower toxicity than harsher solvents. Having worked in a chemistry lab using this solvent, gloves and good ventilation remain standard, but the lower vapor pressure makes the air less rough on the lungs by comparison.
Every good solvent carries a risk of waste and pollution if tossed out carelessly. Once you use 1-Butylpyrrolidin-2-one, there’s a duty to catch and treat residues, through careful capture and controlled incineration. Keeping an eye on environmental impact matters. Studies on biodegradation prove it doesn’t just hang around in the soil or water forever, but responsible disposal always beats optimistic guesswork.
Alternatives appear here and there, yet many lack the same performance at the same cost. Some suggest switching to greener solvents made from plant-based sources, although price and effectiveness remain hurdles. It’s worth pushing for recycled and recovered solvents in industry, making the best use of every drop while developing cleanup systems that catch leaks before they do harm.
In many labs, 1-Butylpyrrolidin-2-one is a reliable partner for chemists cracking tough problems. In my own work, its odor—milder than acetone or traditional amides—ran alongside its power. Handling it felt safer, knowing proper precautions went further than just a whiff test. Beyond the university bench, in industrial plants, engineers bank on it to keep fibers smooth or printed circuits crisp.
Having a handle on the chemical structure lets you understand more than the textbook layout. You see how one compound tricks stubborn materials into solution, why some solvents get top billing, and how simple structural adjustments open new doors. This pays off whether you’re aiming for greener tech or safer workspaces.
The world doesn’t run on water alone. Synthetic solvents fill laboratories, manufacturing plants, and research centers. Some sound exotic, but they all have one thing in common: somebody needs to know how they mix with other substances. 1-Butylpyrrolidin-2-one fits right into this landscape as a lesser-known but increasingly used solvent. It can carry significance for cosmetics, chemicals, pharmaceuticals, and even coatings. What really matters is how it behaves in water and organic media—because solubility makes or breaks how a product is made, stored, or applied.
Solubility in water matters, especially for safety, environmental, and usability reasons. Pouring a new solvent down the drain with the hope it dilutes won’t always work. Many chemical engineers and lab technicians have tried washing glassware only to find a persistent oily film clinging to the sides. 1-Butylpyrrolidin-2-one stands apart from its close relative, N-methyl-2-pyrrolidone (NMP), by being less polar. That longer butyl chain tugs it away from water’s embrace. Peer-reviewed studies and chemical databases show it has limited solubility in water—enough to disperse, but not enough to dissolve like sugar in tea. It's more like trying to stir oil into vinegar; some blending occurs, but a cloudy suspension forms instead of a clear solution.
Move beyond water, and 1-butylpyrrolidin-2-one finds more friends. Chemists swear by organic solvents such as ethanol, acetone, and toluene for dissolving stubborn compounds, paints, coatings, and pharmaceuticals. Here, that long butyl chain shines. The molecule dissolves easily into many less polar liquids. In practice, technicians mix it with acetone, ether, and chloroform, often watching it disappear without a trace. This property gives industries flexibility. Formulators working on drug delivery, agrochemicals, paints, inks, and adhesives reach for solvents like this because they don’t limit creativity the way water does.
Solubility affects more than lab work. Disposal and environmental impact matter. If a solvent doesn’t mix readily with water, it tends to resist natural breakdown and may persist in the environment. That limited water solubility creates challenges for wastewater treatment and increases reliance on incineration or careful recycling. Regulators keep a close eye on these compounds, recognizing that improper handling can harm aquatic ecosystems and human health. People who handle these chemicals every day must think twice before disposing of them casually. Companies look at greener alternatives or better containment whenever possible. Substances that cling to organic solvents but barely touch water need clear protocols and robust education for workers and downstream users.
Manufacturers have options. The push toward safer chemicals with less environmental impact hasn’t slowed, especially in the European Union and United States. Many companies now audit their solvent selections, looking for less toxic, more biodegradable choices. That doesn’t mean abandoning 1-butylpyrrolidin-2-one outright. Instead, teams weigh efficiency and effectiveness against safety and sustainability. Replacing solvents often triggers challenges for product performance, but ongoing research looks to bridge these gaps.
Understanding the interaction between 1-butylpyrrolidin-2-one, water, and organic compounds shapes not only what’s possible on the production floor but also what’s responsible in modern chemical stewardship. Those who work with it know that solubility tells more than a technical story—it shapes real-world choices every day.
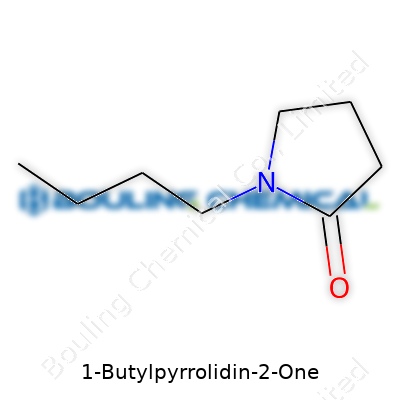
| Names | |
| Preferred IUPAC name | 1-butylpyrrolidin-2-one |
| Pronunciation | /ˈwʌn ˈbjuːtɪl pɪˌrɒlɪˈdiːn əˈwɒn/ |
| Identifiers | |
| CAS Number | 3470-98-2 |
| Beilstein Reference | 1718732 |
| ChEBI | CHEBI:5135 |
| ChEMBL | CHEMBL14326 |
| ChemSpider | 151485 |
| DrugBank | DB08209 |
| ECHA InfoCard | 13d6a292-464d-4e76-a8e3-33f34c4d5ad8 |
| EC Number | 220-763-8 |
| Gmelin Reference | 124515 |
| KEGG | C06936 |
| MeSH | D016248 |
| PubChem CID | 7300 |
| RTECS number | UB2975000 |
| UNII | 9M3K21877D |
| UN number | UN3272 |
| Properties | |
| Chemical formula | C8H15NO |
| Molar mass | 141.22 g/mol |
| Appearance | Colorless to light yellow liquid |
| Odor | Odorless |
| Density | 0.975 g/cm3 |
| Solubility in water | Miscible |
| log P | -0.46 |
| Vapor pressure | 0.0133 hPa (20 °C) |
| Acidity (pKa) | 19.9 |
| Basicity (pKb) | -0.29 |
| Magnetic susceptibility (χ) | -7.52×10⁻⁷ |
| Refractive index (nD) | 1.457 |
| Viscosity | 70.3 cP (25°C) |
| Dipole moment | 4.09 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 362.7 J⁄mol·K |
| Std enthalpy of formation (ΔfH⦵298) | -460.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3996.7 kJ/mol |
| Pharmacology | |
| ATC code | Not assigned |
| Hazards | |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P280-P305+P351+P338-P337+P313 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | 93°C |
| Autoignition temperature | 355°C |
| Explosive limits | Explosive limits: 1.1–6.6% (V) |
| Lethal dose or concentration | LD50 oral rat 3200 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 3,870 mg/kg |
| NIOSH | WH7400000 |
| REL (Recommended) | 5 mg/m³ |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
N-Methyl-2-pyrrolidone Pyrrolidone Pyrrolidine 2-Pyrrolidone |