Chemists first brought imidazole compounds into the lab in the mid-1800s, mostly driven by curiosity about nitrogen’s role in small-ring heterocycles. By the time 1-butylimidazole started emerging on research radars, organic chemistry had shifted from discovery to application: the goal moved from “What is this?” to “What can this do?” The push for efficient catalysts, better corrosion inhibitors, and alternatives to older, sometimes dangerous solvents set the stage. Early syntheses leaned on simple alkylation reactions, but limited yields and tough purification slowed real adoption. Only by late last century did industry find reliable processes that gave chemists a clear route, consistent product, and a reason to consider this liquid for more than a bench curiosity.
1-Butylimidazole lands as a clear, colorless to slightly yellow liquid, usually bottled in dark glass to dodge any photochemical headaches during storage. You’ll spot it labeled as N-butyl imidazole or 1-(Butyl)-1H-imidazole, sometimes shortened to BuImz in research settings. It’s sold for research use, common in both neat and solution form depending on the job to be done. Packaging emphasizes the need to keep it sealed and moisture-free, since too much atmospheric water brings down both purity and shelf life.
On the bench, 1-butylimidazole carries a density just under that of water, floating in at around 0.97 g/cm³. Its boiling point rises above 230°C, making it hardy in high-temp tasks. With a melting point steadily below room temperature, this liquid flows freely through most pipes and flasks—even in chilly labs. Its moderate polarity means 1-butylimidazole dissolves a wide batch of organic salts, but you won’t have much luck with greasy hydrocarbons. Chemically, it stands out by resisting standard oxidants and bases, but does react smoothly with strong acids, alkyl halides, and sulfonating agents. Not a compound you want in contact with open flame, since decomposition can send unsavory fumes into the air.
Bottles fresh from suppliers usually carry assay numbers above 98%. Labels cover CAS number 1003-07-2, keep a GHS pictogram in clear view, and note imidazole odors that some describe as slightly nutty, others as straight chemical. Data sheets don’t skip over pH sensitivity and recommend single-use pipettes to avoid cross-contamination. Standard storage stretches up to two years when kept in cool, dry cabinets away from direct sunlight. MSDS files flag it as an irritant—one splash on skin makes you remember your lab coat.
Alkylation defines the go-to route for 1-butylimidazole, starting from imidazole and n-butyl bromide under gentle heat and in polar solvents like DMF. An experienced chemist uses a base like potassium carbonate to mop up hydrobromic acid as it forms, steering the reaction toward the N1 position instead of an unwanted mix. Extraction with water and organic solvents, followed by distillation, puts a pure product in the bottle. Side reactions threaten if water sneaks in, slamming yields and flavoring the batch with impurities that slow down any downstream chemistry.
1-Butylimidazole serves as a handle for further transformation: it takes on alkyl, acyl, and sulfonyl groups eager to attach to either ring or chain. Chemists count on its nitrogen to serve as a mild nucleophile, so quaternization with methyl iodide or reaction with sulfonyl chlorides can run without drama. The imidazole ring soaks up electron-withdrawing groups, giving rise to charged ionic liquids prized for catalysis or electrochemistry. Reactions generally favor mild conditions—strong acids or halogenating agents lead to decomposition or unwanted tarry messes.
Beyond its formal name, catalogs sort 1-butylimidazole under headings like N-butyl imidazole, 1-(n-butyl)imidazole, or plain BuIm. Pharmacy lists use the full IUPAC structure, Chemistry Source or Sigma-Aldrich stock by product number. Sometimes, confusion with similar alkylimidazoles pops up, so double-checking the structure avoids shipping the wrong bottle for a critical synthesis.
Nobody enjoys chemical burns or respiratory irritation, even in small doses. 1-Butylimidazole splashes sting and carry enough vapor pressure to burn eyes or nasal passages. Industry standards push fume hoods as a must, not a luxury. Gloves—usually nitrile—block direct contact, but most labs keep eye stations and wash bottles within arm’s reach. Fire hazards rank moderate, with recommendations for CO2 extinguishers and routine leak checks. Transport follows standard rules for mild hazards, yet disposal walks a careful balance: dilute to safe concentrations or use licensed solvent waste contractors instead of pouring anything down the drain.
Industry shifted to 1-butylimidazole after finding common solvents fell short, especially in catalytic systems or as ionic liquid building blocks. Electroplating outfits value its chemical stability and ability to dissolve metal salts, giving them brighter, more even finishes. Organic chemists keep it on hand for alkylation or as a base, trading on its selective reactivity and clean-up simplicity. Battery manufacturers look for modifications leading to ionic liquids, which keep electrolytes working even under high voltages or extreme temperatures. Pharmaceutical teams have poked at its potential in drug synthesis, although toxicity keeps it as a tool, not a target. Corrosion inhibition and anti-static coatings round out the commercial crowd.
Labs keep tuning 1-butylimidazole, aiming for greener syntheses or novel properties. Researchers tinker with fluorinated analogues or swap the butyl for longer or kinked chains, hoping to push conductivity or cut toxicity. Combinatorial chemistry has linked it to drug scaffolds, where the imidazole ring’s biological relevance stays alluring. Quantum chemists run computer models to predict its impact on ionic conductivity, while process chemists hunt for low-energy, less wasteful routes. Among start-ups, chatter grows about recyclable solvents that could replace volatile organics with stable imidazole derivatives, keeping pollution down and worker safety up.
Toxicologists approach 1-butylimidazole with healthy skepticism. Data suggests low acute toxicity via dermal or inhalation routes, but repeated exposure produces noticeable irritation and potential liver strain. Animal studies trace metabolic breakdown mostly to the liver, with excretion in urine. No links to carcinogenicity have held up in tests, but researchers keep watch for chronic effects or metabolite accumulation. Manufacturers urge full PPE and ventilation whenever handling bulk quantities and keep “no eating or drinking in the lab” carved in every breakroom sign.
Looking ahead, growth hangs not on raw chemistry, but the need for safe, reusable, and less environmentally damaging alternatives to classic solvents. With regulatory eyes on hazardous air pollutants and waste streams, 1-butylimidazole’s mileage depends on smart engineering, toxicity mitigation, and continuous testing. As batteries, photovoltaics, and specialty catalysis demand more robust functional liquids, this imidazole’s adaptability may keep it near the heart of future labs—provided safety outpaces speed and sustainable sourcing stays more than a buzzword. Industry’s best bet: work with nature, not against it, and squeeze every possible benefit from every carbon and nitrogen in the ring.
Ask someone at a chemical plant about tricky chemicals, and chances are you'll hear about 1-butylimidazole. It’s a colorless liquid, not much to look at, but it pulls its weight in so many jobs that its dull name hides an impressive résumé. You probably don’t run into it in daily life, but if you work in chemistry or the oil industry, you’ve probably used it or seen its name on a barrel.
Factories using copper, nickel, or silver often need those metals squeaky clean for processes like plating. 1-butylimidazole helps remove stubborn tarnish and keeps surfaces smooth, which matters a lot if you want coatings to stick. Companies trust this stuff because it has a knack for grabbing hold of metal ions, preventing them from forming ugly spots or causing chemical hiccups in expensive machines. That efficiency keeps equipment humming and means fewer breakdowns, which saves on repair costs and headaches.
Catalysts turn slow chemical reactions into fast ones, saving time and increasing productivity across industries from petrochemicals to food production. 1-butylimidazole joins forces with metals to create new catalysts that last longer and work at lower temperatures. This saves both energy and money. Chemists have told stories about tired old reactors breathing new life after a tweak that included imidazoles—proof that sometimes the difference between profit and waste comes down to which chemical solutions you reach for.
Some liquids just can’t handle the tough jobs—rusty pipelines, sticky chemical residues, or those mystery gunk buildups in labs. 1-butylimidazole steps up as a strong, reliable solvent, especially helpful in challenging cleaning tasks. It dissolves things that water or weaker solvents leave behind. Plenty of labs keep a small drum of it around for exactly those reasons, and stories float among technicians about finishing up Friday shifts early thanks to its quick work.
The world is getting louder about green solutions, and 1-butylimidazole gets pulled into that conversation, too. Pair it with the right acids or salts, and you get ionic liquids—solvents that don’t evaporate and pollute the air like others. These ionic liquids have found their way into everything from battery research to environmental cleanup. While traditional solvents make a mess of the air, these newer options cut down on fumes and make workplaces safer. The hope is that smarter use of these chemicals will ease the worst impacts of industrial pollution.
Every chemical that helps industry run smooth also brings safety concerns. Workers handling 1-butylimidazole need decent ventilation, gloves, and good training, because exposure can irritate skin and lungs. There’s also the question of waste streams and what gets left behind in soil or water. Regulators want to see tighter rules about how companies use and dispose of the stuff. Firms that plan ahead—using enclosed systems and good recycling methods—avoid fines and protect both their staff and their neighborhoods.
There’s always a new process, a fresh tweak, or a better safety measure waiting just around the corner. Chemists and engineers trading ideas about 1-butylimidazole tend to focus on pushing technology further—stronger materials, faster reactions, cleaner disposal. The people who handle this stuff every day know that in industry, progress doesn’t always come in big leaps. Sometimes it’s the small bottle of an overlooked chemical, doing solid work between the headlines, that makes things better for everyone on the job.
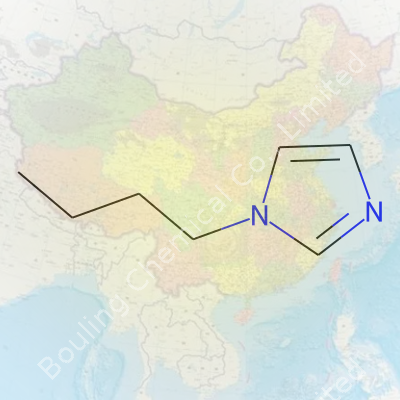
Certain chemical questions draw out genuine curiosity, even from those who never felt a connection with chemistry class. 1-Butylimidazole brings together the organic world and real-world application in a way that’s tough to ignore. This molecule looks humble on paper, but its skeleton carries more than just academic importance. The formula—C7H12N2—tells you what builds it, and the structure offers a hint at why chemists and manufacturers give it attention.
Look at the formula C7H12N2. Here, you’ve got seven carbons, twelve hydrogens, and two nitrogens holding the molecule together. What makes this arrangement practical goes beyond just textbook counting:
Chemical structures aren’t just lines and letters—they decide what you can actually do with a compound. I’ve worked enough with molecules like this to see how just tacking on a butyl group opens up new options in laboratory syntheses. That butyl branch controls solubility and reactivity, often making purification go smoother. Imagine trying to separate a stubborn compound from a reaction mix, and one tiny side chain lets you use a different solvent, saving half a day of wrestling with column chromatography.
Folks interested in green chemistry pay attention to molecules like 1-butylimidazole. By design, these structures slide smoothly into ionic liquid recipes, which are famous for reducing waste and pollution in chemical processes. Factories reusing solvents, researchers recycling their reaction media—these small shifts add up, both for the bottom line and the environment.
Labs and industrial sites face growing heat from environmental concerns. Tough regulations and high disposal costs push everyone to look closer at what chemicals go in, and what comes out. Molecules like 1-butylimidazole offer flexibility. If a process creates less waste or makes solvent recovery simpler, the whole operation runs cleaner and cheaper. Working with chemicals that don’t just evaporate or linger in wastewater cuts down on headaches long after the experiment ends.
Molecular tweaks—like the butyl chain—look minor on paper, but from a bench chemist’s point of view, these changes shape an entire workflow. More than just a line item in a catalog, 1-butylimidazole draws a direct line between smart design in the lab and more responsible manufacturing outside of it. The details in a formula or a ring structure might feel old-school, but for anyone dealing with long hours over a reaction flask, those details make all the difference.
1-Butylimidazole pops up in labs and industries for its unique ability to dissolve certain salts and act as a catalyst. I’ve worked in places where chemical handling is simply a part of life. Clear guidelines and street smarts matter. There’s a tendency for folks to focus just on flammables and acids, but overlooked liquids like 1-Butylimidazole can sneak up on you if you’re not paying attention.
Storing chemicals isn’t glamorous but skipping steps causes problems. 1-Butylimidazole doesn’t like moisture. It absorbs water from the air, which can mess with its purity and affect lab results. Keeping it in a tightly sealed container, preferably glass or high-quality plastic, limits contact with air and water. I’ve tossed out whole batches before because someone left a lid loose on a humid day. Those losses sting, both for the budget and for project deadlines.
The temperature inside your chemical storeroom does more than affect your comfort. High heat leads to pressure build-up or leaks, cold leads to condensation. For 1-Butylimidazole, room temperature in a shaded spot works fine. Don’t stick it next to radiators, sunlight, or cooling vents that swing too cold. Fluctuating temps change the chemistry of some organics without you realizing it.
Every bottle should carry a clear label showing exactly what it is, and the date opened. I’ve seen colleagues pour liquids from unmarked containers thinking they “just knew” what it was. Down that path go ruined experiments, lost data, even accidents. I always mark the cap and the side, so even a rushed coworker can’t miss it.
Ventilation keeps everyone safe. Fumes from 1-Butylimidazole aren’t always obvious, but they build up in unventilated rooms. The slight ammonia scent lingers. Left unchecked, headaches or worse can result. A proper fume hood or exhaust fan makes a big difference. I’d rather spend time opening a window or waiting for the fume hood than risk feeling woozy after a long afternoon.
Gloves and goggles make sense, even if you’ve handled plenty of chemicals before. 1-Butylimidazole can irritate skin and eyes. I know people who thought washing hands after the fact was enough, only to end up with red, burning skin. Always grab gloves, or you’ll remember the sting. If spills happen, clean with lots of water and soap immediately—no shortcuts.
Don’t forget to check for leaks or dripping lids before moving containers. It’s easy to grow casual with routine, only to discover a sticky trail on your bench or coat. It pays to check once more before packing things away.
Disposal is another area that invites carelessness. Pouring leftover chemicals down the drain creates problems. Follow site waste protocols—use special bins for solvents and coordinate with your chemical waste team. Getting rid of 1-Butylimidazole safely avoids headaches for the sewer system and for people downstream.
I’ve found that clear procedures on labeling, smart storage practices, and a no-nonsense approach to PPE make every handling task smoother. Regular spot checks, and having a buddy system to double-check procedures, go a long way. These habits take seconds, but make a big difference in accident rates and product quality. It doesn’t take fancy tech—just people looking out for one another, a good marker pen, and a strict rule about never cutting corners.
Most people would never bump into 1-butylimidazole in their daily lives. For folks working in a lab or chemical plant, though, this compound isn’t just a line on a safety chart. Chemists use it for making ionic liquids, and sometimes as a catalyst. Still, its practical benefits can’t distract anyone from asking: is this stuff actually safe, or does it pose dangers we can’t ignore?
One lesson from working around chemicals is that what you can’t see or smell often causes the most harm. With 1-butylimidazole, you’re not looking at something completely harmless. Letting this liquid near skin can lead to irritation, or even burns with enough contact. If it splashes in eyes, pain and lasting damage surface fast. All of this is just from physical contact—breathing in its fumes starts another set of worries.
Reports show that vapors or mist from this substance tend to irritate the nose, throat, and lungs. In some workspaces, sneezing, coughing, or trouble breathing follows only a short exposure. My experience working in a university research lab taught me that relying on a fume hood felt mandatory, not optional, whenever imidazole compounds came out of the chemical cabinet.
Chemicals don’t just disappear after a quick use. Pouring 1-butylimidazole down the sink, or spilling it outdoors, can reach more than just people. Fish and plant life in water and soil take some pretty hard hits from even small amounts of synthetic chemicals. Studies point to toxicity for aquatic life, especially when it builds up in streams or ponds. Disposal needs real attention: local laws forbid dumping it casually, because it lingers and disrupts more than bacteria or spores in water.
There’s no getting around the risk. So, each lab or company handling 1-butylimidazole needs to face it directly. Gloves resistant to chemicals, safety goggles, and reliable ventilation become part of the routine—not just for show, but as daily protection. Training people how to react after spills or exposure changes statistics from disaster to control. Medical staff should keep up-to-date on treatment options for accidental contact, too.
Labels and documentation serve a bigger role than just filling shelves. Material Safety Data Sheets (MSDS) ought to stay current. These records give clear, non-jargon steps for staying safe, what symptoms to watch for, and how to dispose without harming the water supply. Regulations might feel strict, but these rules came from real injuries and costly mistakes.
The modern push for “green chemistry” tries to scale down the use of harsh solvents and toxic chemicals. Alternatives don’t always work the same way, though. Until safer substitutes come along, respect for chemicals like 1-butylimidazole means thinking ahead. Storage kept away from food or common work areas, clear communication with everyone at risk, and honest hazard alerts become nonnegotiable. After years around research chemicals, I’ve seen how one careless moment can outweigh years of careful planning.
Taking shortcuts with chemical safety never felt worth it—not for me or anyone sharing the space. Informed choices and real communication shift the odds, letting science and industry move forward without ignoring health and safety.
Buyers usually expect a couple of well-defined purity options. In labs and research, 1-butylimidazole often comes at about 97% to 99% purity. Some suppliers call this “analytical grade.” Chemists working on catalyst studies, ionic liquids, or special synthesis usually want as few contaminants as possible. Research projects lean on that higher grade—even trace water or solvents can spoil reactions or skew results. For industrial use, an extra percentage point doesn't always justify higher costs. Some processes absorb the minor impurities. On the other hand, pharmaceutical or electronics industries sometimes demand “ultra-high purity,” where the margin is even tighter, often above 99.5% with detailed impurity profiles by GC, NMR, or HPLC.
I remember pulling a bottle off the shelf marked ‘99%’, thinking it was overkill. It wasn’t. Low-level amine or metal residues can change how 1-butylimidazole behaves with catalysts or sensitive organics. That’s a lesson you learn once, never forget. Re-purifying a liter by distillation isn’t fun—especially if you started with the wrong grade.
Packaging really splits between small-use lab bottles and industrial drums. For bench chemists, 1-butylimidazole turns up in amber glass or sometimes fluorinated HDPE bottles, usually ranging from 25 mL to 1000 mL. Glass keeps light and extra air out. Stores smell down too, something any researcher will appreciate after knocking over a bottle once.
In production, larger players pack it in 5-liter to 200-liter containers, often HDPE drums or cans lined with specialty polymers. Corrosion and reactions with the container matter, especially if you're storing the stuff for a few months. A typical reaction plant has those blue HDPE jugs with tamper-proof seals. Old hands know even a small leak leads to lost product—and a lingering smell through half the building. Bulk orders sometimes ship in stainless steel IBCs or totes for demanding customers who're worried about trace contamination or need huge volumes at once.
Cost and safety never leave the conversation. Lower grades sometimes cut corners on distillation or filtration, carrying residual acid or water. Improper packaging means the compound can absorb water or even leak impurities, particularly with less stable plastics. More than once, someone has opened a drum to find polymerized gunk instead of a clean liquid. Cleanup isn’t cheap—or safe.
Labs and factories waste far too much money and time with mismatched specs. The solution here isn’t complicated: ask the supplier for both the grade’s full analysis and packaging details. Anyone scaling up from lab to plant should test both quality and behavior on a small scale—preferably before betting the whole project on a new barrel. Written specifications, regular spot-checks, and transparency about packaging help fix most of the classic headaches.
Some countries regulate shipments more strictly, particularly for chemicals classed as hazardous. Tracking where and how these chemicals get stored depends on clear paperwork and barcode systems. Packaging should always match not just the volume, but also how long users plan to store the chemical. Sealed drums with nitrogen backfilling can stop oxidation or absorption, keeping the product stable longer.
Overall, the choice between grades and packs comes down to the job at hand and a little practical foresight. Skipping those steps usually costs more in wasted materials or ruined experiments than anyone expects at first. Smart buyers learn fast: ask detailed questions, push for a better container, and always look at the certificate of analysis before opening anything.
| Names | |
| Preferred IUPAC name | 1-butyl-1H-imidazole |
| Other names |
1-Butylimidazole N-Butylimidazole Imidazole, 1-butyl- |
| Pronunciation | /ˈwʌnˌbjuːtɪlɪˈmɪdəˌzoʊl/ |
| Identifiers | |
| CAS Number | 14359-50-1 |
| Beilstein Reference | 0109365 |
| ChEBI | CHEBI:140217 |
| ChEMBL | CHEMBL141345 |
| ChemSpider | 10453573 |
| DrugBank | DB02646 |
| ECHA InfoCard | 06c7bcd2-1e2e-41f7-ae7a-56f6ee3bb164 |
| EC Number | 246-995-8 |
| Gmelin Reference | 82885 |
| KEGG | C19279 |
| MeSH | D000072232 |
| PubChem CID | 75456 |
| RTECS number | NX8925000 |
| UNII | B2A1J1UM75 |
| UN number | UN2810 |
| Properties | |
| Chemical formula | C7H12N2 |
| Molar mass | 138.20 g/mol |
| Appearance | Colorless to light yellow liquid |
| Odor | Ammonia-like |
| Density | D=0.99 g/cm3 |
| Solubility in water | soluble |
| log P | 0.64 |
| Vapor pressure | 0.0095 mmHg (25 °C) |
| Acidity (pKa) | pKa = 6.9 |
| Basicity (pKb) | 5.7 |
| Magnetic susceptibility (χ) | -62.0e-6 cm³/mol |
| Refractive index (nD) | 1.488 |
| Viscosity | 3.8 mPa·s (25 °C) |
| Dipole moment | 3.89 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 244.4 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -33.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3284 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Harmful if swallowed. Causes severe skin burns and eye damage. Harmful to aquatic life with long lasting effects. |
| Precautionary statements | P264, P280, P302+P352, P305+P351+P338, P332+P313, P337+P313, P362+P364 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 104°C |
| Autoignition temperature | 415 °C |
| Explosive limits | Explosive limits: 2–12% |
| Lethal dose or concentration | LD50 (oral, rat): 500 mg/kg |
| LD50 (median dose) | LD50 (median dose) of 1-Butylimidazole: "LD50 oral (rat) >2000 mg/kg |
| NIOSH | KK2450000 |
| REL (Recommended) | 0.02 ppm |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
Imidazole 1-Methylimidazole 1-Ethylimidazole 1-Propylimidazole 2-Methylimidazole 1-Butyl-3-methylimidazolium chloride |