The journey of 1-Boc-pyrrolidine stretches back several decades, following the rising demand for simpler synthetic routes in preparing protected amines. Early work focused mainly on finding ways to mask nitrogen’s reactivity in pyrrolidine rings, letting chemists build more complex molecules step by step. The invention of the Boc (tert-butoxycarbonyl) group as a protective layer brought a real shift, offering a relatively mild method for handling nitrogen atoms in organic chemistry. Over the years, this approach escaped the confines of academic settings and landed on the benches of pharmaceutical labs everywhere. The surge of interest came as peptide chemistry evolved, making stable intermediates a prized commodity. Chemists, including me, have watched simple molecules like 1-Boc-pyrrolidine become a linchpin in both medicine development and everyday research tasks.
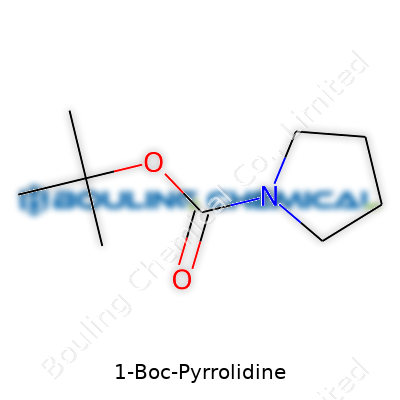
1-Boc-pyrrolidine stands as a protected amine built around the five-membered pyrrolidine ring bearing the Boc group. Companies recognize it under several product codes, but you’ll rarely see a lab catalog without it. I’ve noticed researchers favor this compound for its stable nature and predictable behavior, especially when handling multistep syntheses. For that reason alone, it keeps turning up as an intermediate when creating pharmaceuticals, agrochemicals, and new specialty materials.
This molecule appears as a colorless to pale yellow liquid, sometimes crystallizing at lower temperatures. Its molecular formula is C9H17NO2 and it packs a molar mass near 171.24 g/mol. Its boiling point hovers around 92–93°C at reduced pressure, letting labs purify it easily. The Boc group itself shields the nitrogen from strong acids and bases under most lab conditions, so reactions involving 1-Boc-pyrrolidine rarely end up unmanageable. Its mild odor and low volatility cut down on unwanted exposure. I’ve worked with plenty of amines, some of them pungent and fussy, but 1-Boc-pyrrolidine usually behaves predictably, making life much easier in the lab.
Manufacturers usually offer 1-Boc-pyrrolidine in high-purity grades, often exceeding 98% purity. Standard packaging comes in amber bottles or sealed ampoules, keeping air and light out. Labels list details like CAS number (79915-73-4), batch number, and storage temperature, typically just below room temperature. Safety notices warn about mild irritancy and urge the use of gloves and goggles. Labs track their vials closely, since substitution or contamination can throw off entire reaction sequences. Attention to detail saves time and frustration, as I’ve learned more than once over the course of a career working with protected nitrogen systems.
The usual synthetic pathway starts by taking pyrrolidine and reacting it with di-tert-butyl dicarbonate (Boc2O) under basic conditions. An organic base like triethylamine gets added to neutralize any protons released, and the entire reaction bath handles well below 0°C to avoid side products. The workup steps generally involve standard aqueous washes and a round of drying before collecting the final product. Over time, improvements in solvents and mixing speeds have cut down on waste and improved overall yield. I’ve definitely benefited from these streamlined protocols, as they let me focus on the interesting transformations rather than endless purification work.
The Boc group blocks harsh agents, so chemists can carry out a wide range of reactions elsewhere on the molecule without disturbing the nitrogen. Reductive alkylation works neatly. You get a good yield with modest amounts of aldehydes or ketones. Once modifications wrap up, mildly acidic environments strip off the Boc group, revealing the free amine. In my own projects, I’ve repeatedly used this approach to introduce selective substituents, with 1-Boc-pyrrolidine acting as a launchpad for building more elaborate, functionalized heterocycles.
Suppliers use several names for this compound: tert-butyl 2,3,4,5-tetrahydropyrrole-1-carboxylate, Boc-pyrrolidine, and N-Boc-pyrrolidine all refer to the same structure. Chemical databases, such as PubChem and ChemSpider, use CAS code 79915-73-4 as a reference point, which helps avoid confusion when sourcing. Industry catalogs throw around abbreviations like TBP or NBP, so looking up the CAS registry makes sorting samples much simpler in any busy workspace.
Boc-protected amines like this one require respectful handling but rarely present dangerous hazards. Standard laboratory PPE suffices—lab coats, gloves, basic splash goggles. Material Safety Data Sheets warn against ingestion, skin contact, or eye exposure, and recommend working in well-ventilated areas. Accidental spills wipe up with absorbent pads, with no special disposal requirements beyond normal organic waste protocols. I’ve seen newer lab techs worry about protected amines, but 1-Boc-pyrrolidine rarely leads to panic situations if basic rules are followed. Routine air monitoring in manufacturing sites finds low vapor emissions.
Pharmaceutical development drives much of the demand for 1-Boc-pyrrolidine, particularly for its role as a building block in synthesizing active pharmaceutical ingredients. The peptide sector leans heavily on this compound, as the Boc group lets researchers fine-tune amine reactivity at will. Its uses extend to agrochemical discovery, where new pest control agents often begin as simple amines like this, then graduate to more complex derivatives. Materials science teams have also harnessed Boc-protected pyrrolidines to create everything from molecular switches to diagnostic agents. Over the years, I’ve met colleagues in these fields who swear by the reliability and adaptability of this single intermediate.
Continuous innovation in synthetic chemistry keeps raising the bar for intermediates like 1-Boc-pyrrolidine. Labs have built high-throughput screening workflows that rely on this compound as a starting material. Green chemistry initiatives have sought less wasteful ways to introduce or remove the Boc group, driving down cost and environmental impact. Interest in automated synthesis means suppliers have started offering pre-weighed, sealed vials for machine-driven workflows. In my own experience, switching from bulk bottles to single-use ampoules saves hours of weighing and measuring, preventing cross contamination and boosting overall efficiency.
Toxicology screens show that 1-Boc-pyrrolidine tends to be far less dangerous than unprotected pyrrolidine. The Boc group substantially reduces bioavailability, limiting how much gets absorbed if accidental exposure takes place. In rodent studies, high doses can cause mild central nervous system effects, but the typical laboratory or production worker faces much lower risks. Periodic reviews scan for any genotoxic or mutagenic activity and so far, no red flags have emerged. That said, long-term impacts aren’t completely settled, so regular health monitoring helps catch issues before they escalate. My preference leans toward minimizing skin and inhalation contact wherever possible, regardless of what the data shows, since decades of work in chemistry have taught me that unforeseen risks pop up when least expected.
Looking ahead, 1-Boc-pyrrolidine’s reputation as a reliable intermediate seems solid. Emerging trends, such as miniaturized drug manufacturing and flow chemistry, demand stable, easy-to-handle building blocks. Automation, particularly in pharmaceutical R&D, is driving up requirements for well-characterized, pure materials with minimal side reactions. Advances in green solvent systems and recyclable protecting groups may nudge some labs to try out alternatives to Boc, but for now its market share remains secure. Peptide synthesis, combinatorial chemistry, and fine chemical development show no signs of dropping 1-Boc-pyrrolidine from their roster. If anything, efforts to embrace more sustainable chemistry will only improve manufacturing and handling practices, letting the next generation of chemists benefit from an even safer, cleaner work environment. I see this molecule sticking around, both in research and commercial settings, thanks to its reliability and flexibility.
Ask any chemist, and they’ll tell you the importance of purity in their day-to-day work. Take 1-Boc-Pyrrolidine. The world probably doesn’t turn on the purity of this one molecule, but it sure makes a difference in a lab or production run, especially in pharmaceutical research. Most suppliers offer it in purity ranging from 97% to 99%, with the highest grades tested and documented right on the certificate of analysis. For somebody handling multi-step syntheses, those last few percentage points stand between a smooth downstream process and a mess nobody wants to troubleshoot.
Impurities sneak up on you. They usually look like a small percentage on a specs sheet but wind up in the reaction flask, gumming up yields, or even introducing side-products that nobody anticipated. In pharmaceutical or materials science, it only takes a whisper of impurity to force re-purification, scrap a batch, or put a whole research project on hold. High purity cuts down on surprises. More than once, I’ve seen colleagues spend hours combing through reaction mixtures by NMR, only to learn an off-spec starting material set them back a week.
Anybody checking a product page for 1-Boc-Pyrrolidine sees the classic “99%” or “98% min” tag slapped on. That label isn’t handed out just because a lab technician took a guess. Most reputable suppliers confirm purity with HPLC, GC, or NMR, publishing numbers for anybody who asks. Yet, different suppliers can run tests a bit differently. One operator’s “99% GC” might turn into “97% NMR”—a reminder to always look for the actual data, not just a big bold number.
Shipping and storage matter, too. Even a golden batch degrades under poor conditions, especially with small, reactive amines. Last winter, a shipment turned up at the lab after getting stuck at customs under questionable conditions. Re-tested purity dropped by almost a point—which, on a tight project timeline, ended up costing far more than the premium for a fresh, high-purity sample. Moisture, heat, air—none of them are friends to this compound.
Some buyers try to save a little money on research chemicals, going for “technical grade” or just picking whatever’s in stock. Maybe that works for a quick proof-of-concept, but it rarely pays off in the long run. No researcher I know enjoys debugging a failed reaction because a supplier changed sources, skipped extra purification, or sent leftovers from a previous manufacturing lot.
Labs without access to proper purity analytics wind up relying on good supplier relationships and clear contracts. Whenever the stakes rise—for example, scaling up to pilot runs or prepping for preclinical work—the only practical solution is tighter specs, more frequent testing, and a willingness to reject lots that just aren’t up to snuff. This isn’t about perfectionism; it’s about building predictability into systems where the margin for error gets razor-thin.
No crystal ball can guarantee purity, but a few habits make things smoother. Always dig up the actual analysis for each lot, not just the catalog description. Establish clear specifications before you place a big order, especially if you’re going through a distributor. Store chemicals in stable conditions—no backroom shelves or sunlit windowsill stashes—and double check the bottle’s seal and labeling every time.
At the end of the day, purity for compounds like 1-Boc-Pyrrolidine isn’t just a number. It’s the foundation for work that demands clarity, reliability, and respect for the details that pile up day after day in the world of research.
A lot of labs treat every reagent like another bottle in the fridge. Toss it on the shelf, scribble a date, hope for the best. That habit might work for vinegar or even some solvents, but not for something like 1-Boc-Pyrrolidine. This compound keeps a little secret: it doesn't last long when left out in the open.
1-Boc-Pyrrolidine belongs to the world of nitrogen heterocycles—not just a trivia nugget, but a cue to watch how its structure reacts under the wrong roof. The Boc in its name stands for tert-butoxycarbonyl, a protecting group well-known in peptide chemistry. Leave a bottle open near any damp sink, and the Boc group won’t stick around for the party. Moisture loves to sneak in and ruin things, breaking bonds or kicking off unwanted reactions. Chemists hunting for purity consider this level of risk serious.
A trusted guide isn’t always a web search; sometimes it’s the senior grad student who’s already scrubbed goo off flasks at 2 a.m. The word is: temperature matters. Store 1-Boc-Pyrrolidine in a cool, dry spot. That means away from radiators, sunlight, or any spot where the A/C fails in July. Play with room temperatures and you end up with decomposition, not a clean batch. If you’ve ever worked through product breakdown, you know the pain—risky NMR readings, lost time, wasted money.
Grabbing reagents with wet gloves or forgetting to close bottles adds up. With sensitive organics, it pays off to seal containers well. Vacuum-sealing or using an inert gas blanket isn’t just show-off science; these steps stop air and water vapor from creeping in. In busy labs, one open container can cause a cascade of headaches, especially with stuff that reacts just because it smelled some moisture in the air.
Mixing storage for all reagents saves space but breeds problems. Some chemicals hate their neighbors. Acids or bases sitting too close spell trouble for Boc-protected amines. Storing 1-Boc-Pyrrolidine in a place where it won’t suffer acid fumes or volatile compounds saves money in the long run—less spoilage, fewer failed reactions, no guessing why starting material didn’t behave.
Every bottle earns its sticky label with dates, supplier, and any weird notes from previous users. I’ve opened chemicals with two-year-old expiration dates, and the mess is never pretty. Checking in on a bottle before pulling it for a synthesis helps dodge disappointment. Think of it as reading a milk carton—a bad batch brings headaches, not just for the guy making the solution but for the next three users too.
Take storage seriously, and you stretch every gram of 1-Boc-Pyrrolidine to the max. Work in dry, cool areas. Reseal bottles right after use. Pick a storage spot that won't change humidity or temperature daily. Small habits in the lab keep bigger problems off the to-do list, and that’s something every chemist—the morning people and night owls—learns to appreciate before long.
Step into the world of organic synthesis, and it isn’t long before 1-Boc-Pyrrolidine comes up. At first glance, the name sounds straight from a dusty textbook. Turns out, this molecule pulls quite a bit of weight in research labs and pharmaceutical companies everywhere. I remember my own lab days, hunting through reagent shelves, and spotting those familiar white bottles—more tools of the trade than abstract concepts.
1-Boc-Pyrrolidine does one thing extremely well—it shields the reactive amine group of pyrrolidine. The “Boc” part stands for tert-butyloxycarbonyl, a popular protective group that blocks reactions you don’t want while building a new molecule. If you’ve ever tried piecing together a complex structure, you know a single unguarded amine can derail your whole plan. A chemist adds the Boc group to keep that nitrogen atom out of trouble, pushing the reaction forward in the direction intended.
Pharmaceuticals rely on fiddly, multi-step syntheses. 1-Boc-Pyrrolidine plays a huge part in making new drugs, especially those involving complicated nitrogen-containing rings. I saw colleagues experiment with these derivatives to get new antiviral agents, painkillers, or even experimental psychiatric drugs. The Boc-protected version passes through various reaction conditions that would otherwise mess up the nitrogen atom. Only right at the end, after all the hard work, does the chemist strip off the Boc group to reveal the active amine.
Drug discovery rarely follows a straight line. Researchers test out hundreds of combinations before landing on something useful. Here, 1-Boc-Pyrrolidine keeps popping up—precursor for novel scaffolds, quick route to new analogues, backbone for tweaking molecular properties. Its reliability means less time troubleshooting side reactions and more time exploring new candidates.
This chemistry isn’t just about pills and vials. Outside the pharmaceutical industry, 1-Boc-Pyrrolidine supports the design of fine chemicals and certain agrochemicals that improve crop protection. Research teams rely on Boc protection when tailoring the properties of products designed for the field. Heat, humidity, and all the rough-and-tumble of large-scale agriculture won’t shake the Boc group off until wanted, which avoids wasted material and unnecessary costs.
I remember reading how green chemistry has changed the way chemists look at protecting groups. Old-school reagents often created a mess, but 1-Boc-Pyrrolidine, paired with careful planning, tends to minimize byproducts and hazardous waste. Labs feel pressure to show not just results but also how they get there without burdening the environment. A reliable, easy-on, easy-off protection like Boc fits well with these goals.
1-Boc-Pyrrolidine serves real, practical needs in countless research and production settings. For many of us, it represents a piece of chemistry’s toolkit that just plain works—guarding against problems, opening doors to new medicines, and making sure reactions go the way we want. I know that as long as molecules drive innovation, bottles of 1-Boc-Pyrrolidine won’t gather dust on those shelves.
I’ve met my share of chemists who say, “Just punch the compound into the computer and move on.” This shortcut skips over a deeper benefit. Anyone who’s mixed up a batch of 1-Boc-Pyrrolidine knows you need the numbers right. The molecular weight, 171.24 g/mol, isn’t just trivia. It’s the passport for calculations, the bridge from theory to an actual reaction flask.
Back at university, nobody bothered to mention how one miscalculation would sabotage a full day in the lab. I miscalculated a molecular weight once, assumed a smaller margin of error, and ended up throwing out a pricey batch of reagents. In the real world, the cost of a wrong number hurts a lot more than just your pride.
Let’s cut through the mystique. In both academic and industrial labs, 1-Boc-Pyrrolidine shows up as a flexible building block. Folks making new drugs reach for this one a lot, mainly for its usefulness in protecting the nitrogen atom during synthesis. Weighing out the right amount of this compound means looking up that 171.24 g/mol figure every time. No calculation, no reaction, no product. Simple.
Mistake the molecular weight, you end up with too much or too little reagent. A reaction underperforms or doesn’t finish. Running a pharmaceutical synthesis on a scale of kilos, these small math glitches balloon into expensive failures. A few grams here might not sound like much, but errors stack up and hit timelines or budgets hard.
This isn’t just theory for classrooms or textbooks. Reports from manufacturing plants routinely cite the accuracy of stoichiometric math as a top reason for batch inconsistencies. A study published through ACS in 2021 found that minor calculation errors caused nearly 30% of process deviations in smaller active pharmaceutical ingredient facilities. Things could be even worse in places without strict double-checking protocols.
I’ve seen chemists faced with “unexpected” side-products chase their tails for days, only to realize it all traced back to one overlooked decimal place in molecular weight. Automation helps, but the human side can’t vanish—especially if the starting data is wrong.
One easy solution? Standardize how you check and double-check numbers. Lab teams benefit from a shared database with all the key details, reviewed and signed off by more than one person. Some groups keep laminated quick-reference cards posted above the balances. Others insist no scale moves until a second set of eyes confirms every stoichiometry sheet.
Digital tools have come a long way. Most labs now rely on chemical inventory programs that tie molecular weights to barcodes and sample IDs. These tools are only as strong as the data entered, though. An error typed in once sticks around until someone notices. It pays off to have someone on the team who understands how small missteps in calculation lead straight to big headaches down the production line.
The weight of 1-Boc-Pyrrolidine may just look like a number, but every project’s success depends on folks in the lab respecting the details. The lessons learned from an overlooked decimal place stick with you for years after cleaning up a ruined batch. There’s no replacement for care and discipline—both in the math and in the way teams handle their chemistry.
Walking into any organic chemistry lab, I see shelves packed with reagents that help fuel research or manufacturing. For me, 1-Boc-pyrrolidine brings back memories of summer days hunched over flasks, the sharp tang of solvents in the air, and a clock ticking away as I watched reactions bubble and change color. This compound, with its handy protective Boc group strapped onto pyrrolidine, keeps turning up in projects from pharmaceutical synthesis to agrochemical tinkering. It’s not just another random chemical — people genuinely look for efficient supplies.
Sometimes, a gram bottle is enough for basic research. Move into the commercial world—scale up batches, crank out intermediates, and you hit the wall: sourcing. What I’ve seen, calling up bulk suppliers, is that 1-Boc-pyrrolidine isn’t exactly hiding. Over the last five years, several Chinese firms launched catalogs with everything from 100 grams to 50-kilogram packs available for order. US and European specialty chemical providers do ship large containers, but prices jump thanks to shipping fees and local regulation. It isn’t like buying sugar from the grocery, though. Purity, storage, and paperwork change everything.
From what I’ve learned negotiating with suppliers, timing is a real pain. Sourcing hundreds of kilos takes more than filling out a web order form. Certain suppliers want to know end-use. Screening for controlled substances slows things. You wait for stock checks, background reviews, and sometimes, urgent projects stall for weeks. Seasoned colleagues warned me: confirmed delivery schedules don’t always hold up, especially if global shipping gets thrown off by a port closure or regulatory hiccup.
Another snag shows up in quality. Even trusted suppliers sometimes ship batches with purity hovering around 97%, not the 99+% some pharma syntheses require. Testing every lot in-house chews up time and money. One bad batch hit our pilot program—wasted solvents, delayed timelines, and far too much coffee while troubleshooting.
With strong generic drug demand and new startups dipping into small-molecule synthesis, sellers know there’s steady business. They set minimum order levels and rarely budge on price, especially for smaller firms without standing contracts. Having worked as a go-between for a startup I watched budgets melt away thanks to fluctuating quotations and sudden “rush order” fees. Market price updates often lag, meaning you can get stung by a sudden surge.
Based on years of chasing down hard-to-find chemicals, I learned that clear, early communication helps. Lining up bulk purchases isn’t just about clicking “add to cart.” Starting conversations with reps, locking in delivery dates, and confirming specs keeps everything running smoother. Bulk buyers who trust their contacts, negotiate fixed pricing schedules, and check every COA (Certificate of Analysis) catch fewer headaches.
If access dries up or prices climb too high, some teams actually start small-batch synthesis in-house. This choice comes with its own mess—waste, handling, more labor—but for some, faster turnaround trumps all. Having backup supply chains or working together with neighboring labs or CDMOs (Contract Development and Manufacturing Organizations) can also add stability and flexibility to bulk sourcing, especially in shaky markets.
Whether it’s someone in a research lab, an industrial plant manager, or a procurement officer, the story remains the same: reliable access to high-quality, bulk 1-Boc-pyrrolidine can shape timelines, budgets, and even scientific outcomes. In a world where every month another supply chain story breaks, it pays to watch market signals, remember lessons from the last big order, and never take “in stock” at face value without double-checking.
| Names | |
| Preferred IUPAC name | tert-butyl 1-pyrrolidinecarboxylate |
| Other names |
tert-Butyl 1-pyrrolidinecarboxylate N-Boc-pyrrolidine |
| Pronunciation | /wʌn-bɒk-pɪˈrɒlɪdiːn/ |
| Identifiers | |
| CAS Number | 79983-71-4 |
| 3D model (JSmol) | `3D model (JSmol)` string for **1-Boc-Pyrrolidine** (tert-Butyl 1-pyrrolidinecarboxylate): ``` Brc1ccccn1C(=O)OC(C)(C)C ``` *(Note: This is the SMILES string for 1-Boc-Pyrrolidine, suitable for use in JSmol or other molecular viewers.)* |
| Beilstein Reference | 142654 |
| ChEBI | CHEBI:131982 |
| ChEMBL | CHEMBL418236 |
| ChemSpider | 196555 |
| DrugBank | DB14643 |
| ECHA InfoCard | 24-211-975-424 |
| EC Number | 872-85-5 |
| Gmelin Reference | 1262913 |
| KEGG | C10936 |
| MeSH | D017596 |
| PubChem CID | 11449738 |
| RTECS number | UJ4375000 |
| UNII | 9G1326B4K4 |
| UN number | UN3272 |
| Properties | |
| Chemical formula | C9H17NO2 |
| Molar mass | 157.22 g/mol |
| Appearance | White solid |
| Odor | Odorless |
| Density | 0.99 g/cm3 |
| Solubility in water | Slightly soluble |
| log P | -0.01 |
| Acidity (pKa) | 11.3 |
| Basicity (pKb) | pKb ≈ 3.4 |
| Magnetic susceptibility (χ) | -64.92e-6 cm³/mol |
| Refractive index (nD) | 1.450 |
| Dipole moment | 3.28 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 416.2 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | '' |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | P264, P271, P280, P302+P352, P304+P340, P312, P321, P332+P313, P337+P313, P362+P364 |
| NFPA 704 (fire diamond) | 1-2-0 Health:1 Flammability:2 Instability:0 |
| Flash point | 77.5 °C |
| PEL (Permissible) | Not established |
| REL (Recommended) | 200-500 mg |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
1-Boc-piperidine N-Boc-azetidine Boc-proline Boc-pyrrolidin-3-one Boc-piperazine |