In organic chemistry, every compound has a story. 1-Boc-2-Piperidone didn’t just emerge out of nowhere as a handy intermediate for researchers and industry. The piperidone family started gaining attention in the 20th century, as the demand for diverse nitrogen heterocycles grew in tandem with pharmaceutical expansion. Chemists needed stable, manageable forms of reactive piperidones, which triggered the popularity of N-protecting groups like the tert-butoxycarbonyl (Boc). The combination—placing a Boc group on the 2-piperidone ring—offered a clever way to store and manipulate this building block without unwanted side-reactions. Over decades, labs refined each step, learning the quirks of the molecule and pushing it into the wider toolkit we rely on today.
The molecule itself serves as a linchpin for anyone working with alkaloids, peptides, or bespoke pharmaceuticals. Chemists often describe it as a key intermediate; that just means it’s so useful, whole families of drugs or bioactive compounds can start with this puck-shaped, colorless solid. Unlike more volatile reactants, it brings stability to the bench, allowing work to continue without dramas like hydrolysis, oxidation, or degradation. Companies that trade in fine chemicals see steady orders for this compound, sometimes under various synonyms or code names. End users rarely see 1-Boc-2-Piperidone in finished medicines—its job is to keep complex molecules accessible to those whose hands will transform it next.
1-Boc-2-Piperidone looks understated: a white to off-white crystalline solid at room temperature. Its melting point typically settles around 72–76°C, which means gentle conditions suffice for purification. You won’t smell much—low volatility keeps it under the radar. Unlike some protective groups that degrade under mild acid or base, the Boc group’s resilience means 1-Boc-2-Piperidone holds up under standard laboratory atmospheres. Its solubility in most organic solvents, such as dichloromethane, ethyl acetate, or THF, offers flexibility in reaction planning. Chemically, the piperidone ring brings together amide and lactam functionality, giving chemists plenty of levers for transformation.
Bottles arrive from suppliers marked not just with the name but also batch number, CAS code (79099-07-3), purity (often over 98%), and spectrum data so you check for identity and contaminants. Labels warn of irritation risks, and storage guidance usually suggests a cool, dry place, away from moisture and incompatible chemicals. The reliability of technical data becomes vital when you run multiple experiments: melting point ranges, NMR references, and spectral fingerprints reduce the risk of mix-ups in a busy lab. Unambiguous labeling supports safe handling and gives you peace of mind if regulators or collaborators visit your workspace.
The laboratory synthesis starts with 2-piperidone, which reacts with di-tert-butyl dicarbonate in the presence of a base, usually triethylamine or sodium carbonate. The Boc group slips onto the nitrogen smoothly if you control temperature and water exclusion, with purification by recrystallization or flash chromatography afterward. Some larger outfits run this reaction in bulk, but even at small scale, yields climb over 80% under well-optimized conditions. Over time, process development focused on cleaner workups and better atom economies, as tightening regulations and higher scales demanded less waste. For those outside the process lab, it’s still useful to remember that upstream purity and reaction stoichiometry play outsized roles in final product quality.
As an intermediate, 1-Boc-2-Piperidone is famous for versatility. The lactam group acts as a rallying point for nucleophilic attack, ring opening, or alkylation. Remove the Boc with mild acids—trifluoroacetic acid or HCl in dioxane—and you’ve freed up the secondary amine for further diversification. Alkylation or acylation on this nitrogen atom opens the door to building diamines, diketopiperazines, or peptidomimetics. The carbonyl at the 2-position enables reduction, organometallic additions, and cyclization reactions. Medicinal chemists exploit these features to churn out diverse analogues, especially in CNS drug research or antimicrobial scaffold development. The interplay between stability and reactivity here supports lots of creative synthesis beyond the textbook examples.
Even if you ask for 1-Boc-2-Piperidone directly, don’t be surprised to find listings like N-Boc-2-piperidone, tert-Butyloxycarbonyl-2-piperidone, or simply (Boc)-Piperidone. Catalogs might use the CAS number, or abbreviations such as Boc-Pip-2-one. For regulatory tracking, these synonyms run alongside internal codes from suppliers—for example, SDP-1384 or C-190873. Knowing a handful of these aliases helps sidestep confusion, especially when ordering from international suppliers or pulling historic data from older publications. In digital databases, cross-referencing with structural and spectral identifiers (SMILES, InChI) becomes routine for research teams working across continents.
Lab personnel deal with 1-Boc-2-Piperidone using standard PPE—safety glasses, gloves, and lab coats. The compound doesn’t chase after air moisture or burst into flames like some reagents, but sensible handling means limiting skin and respiratory exposure. Fume hoods remain the gold standard during synthesis or weighing, since powdered forms can cause coughing or skin dryness. Spills get wiped up with inert absorbent and disposed of according to local chemical control rules. Written protocols for storage, labeling, and disposal prevent accidents and help new lab members pick up safe habits. Updates on hazard ratings and chronic toxicity refresh any time new regulatory data comes to light—what looked non-problematic yesterday might carry fresh scrutiny after another round of animal testing or environmental review.
Pharmaceutical discovery and manufacturing represent the biggest home for 1-Boc-2-Piperidone. Medicinal chemists put it through its paces in the design of CNS-active agents, analgesics, schizophrenia therapies, and even certain HIV integrase inhibitors. The protected piperidone core makes iterative SAR studies straightforward—swap a group here, tweak a ring there, and you capture a new analogue with different potency or selectivity. In specialty chemicals, it pops up in the preparation of bioactive peptides and peptidomimetics, as its Boc group provides controllable reactivity during solid-phase or solution-phase synthesis. Some patent filings in agrochemicals and material science lay claim to derivatives as well, showing the backbone’s ability to jump between completely distinct scientific fields. Beyond the lab, regulatory folks monitor its potential as a precursor to controlled substances, navigating the fine balance between access for legitimate users and risk management for society.
Every year brings another crop of publications that riff on the piperidone backbone. Researchers in academia and industry reach for 1-Boc-2-Piperidone when developing new peptide-based drugs, next-generation antibiotics, or enzyme inhibitors. The molecule also sparks interest in green chemistry, where teams test solvents, catalysts, and coupling partners that shrink the environmental footprint. Process engineers chase more efficient, safer, and lower-cost synthetic routes, sometimes adapting continuous flow or solid-supported methods for scale-up. Analytical labs refine chromatographic and spectroscopic methods to spot impurities at lower and lower thresholds. As alternate protecting groups and ring systems emerge, researchers hold up 1-Boc-2-Piperidone as a benchmark—any new method runs head-to-head against it in terms of yield, cost, or functional group tolerance.
1-Boc-2-Piperidone usually dodges the most dangerous categories, but that doesn’t make it risk-free. Acute studies report low oral and dermal toxicity in rodents. No sign of mutagenicity or reproductive toxicity has turned up in basic screens, but long-term data remains sparse. Ongoing efforts compare breakdown products and metabolic fate under simulated biological conditions. Since the compound’s primary use sits at the starting line for new pharmaceuticals, regulators expect each derivative to pass its own safety gauntlet—a structure as benign as 1-Boc-2-Piperidone doesn’t automatically mean downstream products will behave the same way. Environmental assessments follow in the wake of larger facility use, scrutinizing rinsewaters and solid waste streams for accidental releases. Staying informed as more studies wrap up helps professionals keep policies rooted in the most credible, up-to-date science.
Looking ahead, the destiny of 1-Boc-2-Piperidone follows the arc of the pharmaceutical and high-value chemical industries. As labs pivot toward more sustainable and targeted synthesis, modifications in the piperidone family—swapping the Boc group or tailoring the ring for better bioactivity—will keep engineers and chemists busy. Digital chemistry and automated synthesis platforms make repeat reactions with this compound more appealing, as high-throughput teams seek robust, reproducible intermediates for big screens. Scrutiny around controlled substance manufacture could reshape the supply chain, ratcheting up documentation and security from shipping to storage. For those in the trenches—academic, industrial, or regulatory—the compound’s endurance signals a healthy intersection between old-school bench chemistry and the new technology shaping the next chapter of molecular innovation.
Chemists spend a lot of energy chasing after pure compounds. I remember the buzz in the lab when someone managed to coax that extra percent of clarity out of a batch. For a molecule like 1-Boc-2-Piperidone, those purity numbers aren’t just a badge of honor—they can make or break an experiment. Ask someone working with medicinal chemistry or peptide synthesis, and you'll hear plenty of stories where impurities threw an entire project off course.
Most labs expect over 98% purity from commercial sources these days. Lower grades risk introducing extra peaks on NMR, ghostly tails in chromatography, and unwanted chemical side reactions. Impurities aren’t just a minor irritation; they shape results, take time to track down, and eat into research budgets. As I’ve seen, even half a percent of “mystery material” alongside your compound means you might waste days troubleshooting failed reactions or, worse, get misleading data.
1-Boc-2-Piperidone’s role as an intermediate means things snowball fast. This molecule gets used in assembling more complicated structures. If someone builds on something impure, it’s like baking bread with flawed flour. That contamination can land in the final product, making quality control downstream a nightmare.
People often trust COAs—certificates of analysis—from suppliers, but experience tells me those numbers aren’t always the full story. HPLC purity can read as 99%, but you might discover by NMR that the batch contains water, traces of solvent, or other unforeseen compounds. Manufacturers and labs shoot for tight specs. Commonly, you see ranges like 98%-99.5% for commercially available 1-Boc-2-piperidone.
In my time with benchwork, I’ve seen how even reputable suppliers can have off days. Fresh batches sometimes show unexpected byproducts, likely formed during synthesis or storage. Checking identity and purity in-house with proper analytical tools—NMR, LC-MS, IR—often saves a lot of frustration down the road.
The drive for high purity isn’t cheap. Producers must choose between more purification steps and lower yields or lower cost and less effort. For researchers, there's pressure to balance budget with performance. Academic labs, strapped for cash, might gamble on cheaper, slightly less pure compounds, but then pay the price in extra work. Industrial settings, especially pharma, absolutely demand high-purity material, as regulations and patient safety demand a tighter ship.
There’s room for improvement. Suppliers get overloaded with price competition and rush orders. More transparency helps, like publishing detailed graphs instead of a single number, or providing real batch-to-batch comparisons. I’ve found that building strong relationships helps—calling up the supplier, asking about batch quality, even swapping notes with fellow chemists online.
Researchers who routinely double-check their samples before big syntheses find fewer problems down the line. Academic programs should push students into the habit early on: if you don’t check your building block, you run the risk of wasting a lot of time, effort, and money.
Chemical purity, including that of 1-Boc-2-Piperidone, reflects many things: care taken in synthesis, competition among suppliers, the pressure of deadlines, and the diligence of the end user. Purity is more than a percentage on a label; it’s a story about everyone in the chain, from production floor to research bench.
Anyone who’s spent a little time working with organic compounds in a lab knows these chemicals come with quirks. 1-Boc-2-Piperidone, for example, fits right into that club. This compound serves as a firm building block for plenty of pharmaceutical syntheses, and you’ll catch its name in research papers focusing on medicinal chemistry. None of that matters if you store it poorly.
I can remember a project in grad school going sideways, all because a white powder ended up absorbing a bit of humidity overnight. The resulting goo was nothing like the substance we’d weighed out the day before. It’s not some one-off story, either. Labs scrap batches of expensive material because someone slipped up on storage, which eventually costs time, effort, and a fair amount of patience.
1-Boc-2-Piperidone lives longer and purer away from strong light and heat sources. Stash the bottle in a refrigerator or a cool cabinet, away from sunlight sneaking through a window. Light can kickstart slow breakdown, and higher temperatures often mean new (and unwanted) side reactions. A chemical fridge set around 2–8°C works well. Avoid that common temptation to leave bottles out on a workbench after use. Just slipping them back into a cool, dark place preserves their fine white powder form.
Humidity turns out to be the silent enemy. This compound holds up fine as long as the cap goes back on tight after each use. Moisture running wild in a storeroom makes for clumpy solids, loss of purity, and even low yields in future reactions. I’ve opened jars where caked bottoms meant tossing grams of perfectly good product. Since most labs feel humid during a rainy season or in certain climates, those silica gel packs tossed in with the bottles earn their keep. They suck up stray water before it touches the chemical.
Every researcher has chased down mystery jars after someone tossed a bottle back on the wrong shelf. Labeling is the low-hanging fruit here: chemical name, concentration (if it’s in solution), date received, and your initials. That way, others know it’s yours and see if they can trust what’s inside. If something starts to change color or texture, documenting it helps everyone dodge future headaches.
I’ve seen plenty of labs keeping a simple inventory spreadsheet or using QR codes stuck right on bottles. These steps don’t cost much, but save hours in the long run. No more wild goose chases for a compound you could have sworn was there yesterday.
Add 1-Boc-2-Piperidone to the list of compounds that should be handled with care. Spills should be cleaned up right away—powders drift and contaminate everything. Keep the container far from acids and reducing agents. Cross-contamination can prompt unexpected fires or fumes. It tastes bitter in the mouth just thinking about how some carelessness can cost an entire batch.
Improving these small habits boosts everyone’s workflow. Safety gear—gloves and goggles—protects your skin and eyes, especially if handling a bigger batch. The benefits add up fast: fewer ruined reactions, less downtime, and a safer workplace overall.
Caring for 1-Boc-2-Piperidone properly just makes sense. Simple routines, like storing it in a cool, dry place away from sunlight, make all the difference. Tight seals, clear labels, and a dash of organization spare staff far bigger headaches later. Techniques I picked up during lab work apply everywhere: a little care on the front end saves money, sanity, and future breakthroughs.
A lot of folks jump into the lab with big dreams and a handful of chemicals, but things can go sideways if you don’t know the specifics. One of the first things I learned during my early days in organic chemistry is that knowing the molecular weight of what you’re using isn’t just about passing a quiz. For 1-Boc-2-Piperidone, the magic number you’re looking at is 198.25 g/mol. This isn’t just a data-point; it’s a number that guides every move you make when weighing, reacting, and purifying.
Imagine setting up a reaction and realizing, too late, that calculations are off because you looked up the wrong molecular weight. It messes up yields, wastes reagents, and costs time — all things you want to avoid. I’ve seen seasoned researchers double-check their notepads or even scribble calculations on their gloves before adding anything to a flask. They do it because tiny slip-ups multiply, especially when scaling up.
1-Boc-2-Piperidone shows up a lot, especially for chemists working with peptides or heterocycles. Its structure – a piperidone ring tucked behind a Boc (tert-butoxycarbonyl) group – helps with selective reactions. Boc isn’t just a fancy name, it protects nitrogen atoms and lets you build more complex molecules. This protecting group pushes up the molecular weight, so don’t just assume piperidone's own figure will cut it.
Get the formula right: C10H17NO3. You can count it for yourself: ten carbons, seventeen hydrogens, one nitrogen, three oxygen atoms. Once the calculation is done properly, you land at that 198.25 g/mol. In real lab work, you won’t always have pure compound. Water, solvents, even leftover reactants can change what ends up on the balance. Real world prep means your theoretical 0.5 mmol might be a bit off if you forget to factor in impurities.
I’ve learned the hard way that weighing an oily sample and skipping a moisture check can explain why your yields look strange. Especially with as popular a building block as 1-Boc-2-Piperidone, lab journals fill up with tweaks and lessons learned by grad students and postdocs armed with nothing but a spatula and basic arithmetic.
Most synthetic projects ask for careful stoichiometric planning. Grab the calculator, punch in the molar mass, and figure out how much you’ll need for the job. Going by volume won’t cut it. Pipetting liquids instead of weighing powders might seem faster, but density and accuracy become issues. Trusting the molecular weight lets you keep things tight when matching reactants, scaling up, or troubleshooting why a batch ran off course.
Lab supply companies print molecular weights in their catalogues for a reason. Even the best chemist can’t squint at a white solid and guess what it weighs. Inconsistent results usually track back to simple mistakes: not double checking calculations or using the figure for an unprotected piperidone. Reflection and attention to these numbers build the habits that keep messy surprises at bay.
I look at molecular weights as a foundation block. You don’t have to obsess, but double checking calculations has saved more than one experiment. If you’re new to the bench, slow down and get your numbers right before you reach for the reagent bottle. For seasoned chemists, precision isn’t just a habit – it’s a key to creativity and reliability.
Stories about chemical supply chains rarely get much limelight, yet anyone working in synthetic chemistry knows the name 1-Boc-2-piperidone. It’s a building block, a starting point, a stepping stone for all sorts of research and production—especially in pharmaceutical circles. In my experience rummaging through catalogues or calling up suppliers, I’ve noticed a sharp uptick this past year in people asking for this compound in not just grams, but kilos. Research labs aren’t just tinkering anymore; companies need volume for pilot batches, process development, and sometimes even for active pharmaceutical ingredient (API) routes.
Bigger orders bring bigger headaches. One reason: 1-Boc-2-piperidone has regulatory red tape across many countries. Some call it a “precursor” because it fits into synthesis routes that nobody wants falling into the wrong hands. If you work in a regulated environment, you know the drill—purchase orders don’t always fly through, and paperwork can drag out simple requests.
There’s also the issue of consistency. Small batches from boutique suppliers can show up fine, but scale often means new variables. I’ve seen buyers test five barrels and find odd results in one, leading to suspicion over storage methods, purity, or even counterfeit sources sneaking into the market. The shadow of supply chain reliability always hangs overhead. Chemical companies outside the US or Europe can promise quantities, but buyers still need trust in quality and handling.
Pricing deserves a close look. The difference between a single bottle and a drum is night and day. It’s not just cost per kilogram; it’s also shipping, special permits, and often insurance. Some buyers end up working with middlemen who hike prices even more. In the labs where I’ve worked, budgets often stretch thin before a project even starts. Cheap product can mean questions about purity, and expensive sources squeeze margins when scale-up gets real.
There’s a trickle effect happening—if biotechs and pharma groups can’t pin down a stable, affordable pipeline for these key intermediates, research timelines slip and small startups might walk away from promising projects. I’ve talked to smaller outfits who end up scraping sample quantities together from various sellers, patching what should be a smooth process just to meet internal deadlines.
Some companies form partnerships with bigger suppliers or invest in multi-month contracts, locking in volume ahead of time. Others start approaching custom manufacturers, asking for tailored syntheses at a cost. I’ve seen collaborative networks spring up: universities exchanging supplier tips, shared lot testing, or even group purchasing to leverage buying power. It’s a patchwork solution at best, but for now it’s what works.
Transparency is what most chemists I know crave. Reliable certification, consistent supply, and direct answers from vendors about shipping, purity, and regulatory requirements would make a world of difference. No one wants to spend hours deciphering supplier codes or translating compliance documents.
Anyone counting on 1-Boc-2-piperidone in bulk should plan ahead, do their due diligence, and keep communication lines open with more than one supplier. It’s all about making sure that when the work needs to be done, the raw materials are actually in hand and up to spec. Problems in the supply chain aren’t going away soon, so anyone in the trenches can’t take access for granted. The scramble for scale, quality, and security remains real.
Walk through any medicinal chemistry lab and you’ll probably spot a bottle of 1-Boc-2-Piperidone somewhere on a shelf. Chemists often use this compound as a building block for creating new medicines. Its flexible structure helps researchers piece together all sorts of important molecules, especially those related to neurological disorders or pain management. I’ve seen teams pour over trial after trial, tweaking parts of this molecule to see if it’ll deliver the desired effect in the body. Many potential treatments for depression, schizophrenia, and chronic pain start life thanks to 1-Boc-2-Piperidone providing a reliable foundation.
What sets 1-Boc-2-Piperidone apart isn’t anything fancy at first glance. It’s protected with a Boc group, which sounds technical, but it’s really just a chemical shield. This little feature lets the rest of the molecule stay stable while scientists play around with other chemical groups. That stability means researchers can confidently perform reactions without the thing falling apart. For a chemist trying to build a complicated drug, every step that stays on track means time saved—and fewer headaches all around.
In the real world, every new drug faces a mountain of challenges before it ever reaches a pharmacy shelf. You don’t reach those breakthroughs with fancy equipment alone. Success comes from clever use of basic, reliable ingredients. 1-Boc-2-Piperidone is one of those, feeding into a wide range of synthetic steps. I’ve watched research colleagues use it for morpholine and piperidine derivatives—key players for new antibiotics, anti-viral drugs, and compounds targeting cancer. Making tweaks to that backbone is where many pharmaceutical advancements come from.
Many synthetic steps aren’t as neat as people imagine. Impurities can sneak in, sensitive reactions can get derailed, and yield can disappear if any part goes sideways. I remember working on a project where one unstable step cost our group weeks of delay. 1-Boc-2-Piperidone cuts that risk down. Its protective group can be removed right after its work is done, leaving a pure product behind. Chemists know this trick as “Boc removal,” and it’s a trusted move, almost like having a shortcut in an unfamiliar city.
One area that keeps popping up is the call for greener, safer methods. Traditional routes for using 1-Boc-2-Piperidone sometimes need harsh solvents or expensive reagents. From what I’ve seen, research labs are starting to troubleshoot these steps. Swapping in cleaner ingredients, finding milder reaction settings, and recycling reaction leftovers are all on the table. Even a few small wins here could save a good chunk of money for companies and reduce the headache of hazardous waste.
Anyone tackling the job of drug development knows it rarely goes as planned. Building blocks like 1-Boc-2-Piperidone make hard work easier, but there’s no pause in looking for smarter ways to use it. Open sharing of process improvements helps keep costs down and avoids some recycled mistakes. As more young chemists pick up these methods, we see quicker progress and a smoother path from lab bench to tested medicine. Sometimes the quiet, reliable compounds make the biggest difference—1-Boc-2-Piperidone stands out exactly that way.
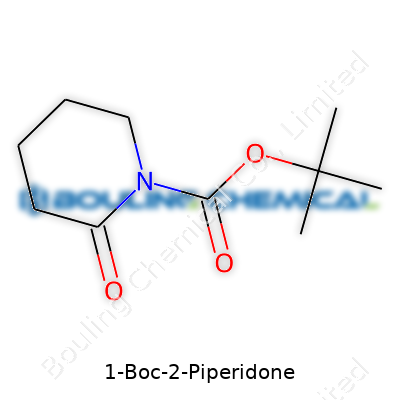
| Names | |
| Preferred IUPAC name | tert-butyl 2-oxopiperidine-1-carboxylate |
| Other names |
tert-Butyl 2-oxopiperidine-1-carboxylate 1-Boc-2-piperidone tert-Butyl 2-oxopiperidinecarboxylate Boc-2-piperidone |
| Pronunciation | /ˈwʌn bɒk tu paɪˈpɛrɪdoʊn/ |
| Identifiers | |
| CAS Number | 79099-07-3 |
| 3D model (JSmol) | `3D model (JSmol)` string for **1-Boc-2-Piperidone**: ``` CC(C)(C)OC(=O)N1CCCC(=O)C1 ``` |
| Beilstein Reference | 89868 |
| ChEBI | CHEBI:189441 |
| ChEMBL | CHEMBL373595 |
| ChemSpider | 10635544 |
| DrugBank | DB08319 |
| ECHA InfoCard | 18a03b44-64b2-49e0-84da-5f14a048dccc |
| EC Number | 6291-35-2 |
| Gmelin Reference | 1261585 |
| KEGG | C19136 |
| MeSH | D000070497 |
| PubChem CID | 160605 |
| RTECS number | YV5950000 |
| UNII | BQN7M64H3M |
| UN number | UN3272 |
| CompTox Dashboard (EPA) | DTXSID80114254 |
| Properties | |
| Chemical formula | C10H17NO3 |
| Molar mass | 199.24 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.08 g/cm³ |
| Solubility in water | Insoluble in water |
| log P | -0.15 |
| Acidity (pKa) | 19.6 |
| Basicity (pKb) | 6.69 |
| Magnetic susceptibility (χ) | -74.5e-6 cm³/mol |
| Refractive index (nD) | 1.491 |
| Viscosity | Viscous oil |
| Dipole moment | 4.20 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 354.4 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -555.6 kJ/mol |
| Pharmacology | |
| ATC code | |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P272, P280, P302+P352, P305+P351+P338, P362+P364, P405, P501 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 117.8 °C |
| NIOSH | Not Listed |
| PEL (Permissible) | Not established |
| REL (Recommended) | 5.0 |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
2-Piperidone N-Boc-piperidine N-Methyl-2-piperidone N-Boc-pyrrolidine 4-Boc-piperidone |