Chemists first explored the structure of 1-Benzylpiperidin-3-ol in the mid-20th century during a broader search for new heterocyclic compounds with possible medical uses. The early days of its discovery echo the era’s enthusiasm for piperidine derivatives, which often popped up in projects aimed at designing antipsychotics, antispasmodics, and other central nervous system agents. Early work on related scaffolds quickly established that tweaking the piperidine ring could lead to profound changes in bioactivity. Over the decades, curiosity about this compound grew, especially as research chemists fine-tuned substitution patterns hoping to unlock better pharmacological profiles and more selective biochemical actions.
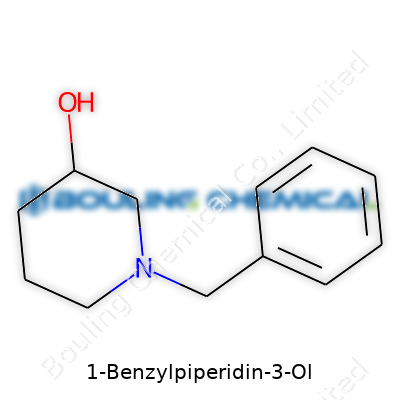
1-Benzylpiperidin-3-ol offers an intriguing mix of chemical stability and reactivity. The molecule features a benzyl group attached to the nitrogen of a six-membered piperidine ring, with a hydroxyl group on the third carbon. That structure works as both a synthetic building block and a part of more elaborate molecules in the medicinal chemistry toolkit. Today, labs value it as an intermediate when pursuing analogs of pharmaceuticals, particularly those targeting central neurotransmitter systems. Unlike some other piperidine derivatives, it balances reactivity and manageability during manipulations, which opens up more versatility on the bench.
In pure form, 1-Benzylpiperidin-3-ol typically presents as a colorless to light yellow oil or crystalline solid, depending on purification and storage conditions. The typical melting point drifts above room temperature, though many batches remain oily at ambient conditions. Chemists pay close attention to its solubility: it dissolves easily in most non-polar organic solvents, yet shows enough polarity to blend with alcohols and ether. The hydroxyl group confers some hydrogen bonding, bringing modest water solubility. Yields in synthesis often swing with subtle pH and temperature changes, a nod to its sensitive ring-and-side-chain combination. On the molecular scale, it weighs 205.29 g/mol and appears in NMR and IR spectra with distinct signals for the aromatic, aliphatic, and hydroxyl regions.
Reliable sources standardize assays for 1-Benzylpiperidin-3-ol above 98% purity. That’s significant—not just for reactions—but for regulatory filings and scientific reproducibility. Labels list the full systematic name, CAS number, batch code, molecular formula (C12H17NO), and typical storage suggestions. Most suppliers recommend cool, dry, and dark conditions with tightly sealed containers, not only to avoid moisture uptake but also to slow down any oxidation. Packing includes hazard pictograms as outlined by GHS standards, given the mild toxicity and low-level irritation risk, which keeps lab accidents in check.
Benzylation and ring functionalization remain the backbone of most synthetic routes. The most robust path kicks off by reacting piperidin-3-ol with benzyl chloride under strongly basic conditions, typically in an aprotic solvent like DMF. Careful temperature control avoids side reactions and over-alkylation, as uncontrolled runs can generate dibenzylated or degraded by-products. In my own time in small-scale synthesis, using sodium hydride as a base improved selectivity. Alternatively, catalytic hydrogenation of 1-benzyl-3-ketopiperidine, followed by a gentle reduction, can deliver high yields where sensitive side groups need protection. Each approach hinges on tight monitoring of pH and extraction steps, given the water solubility sometimes introduced by the alcohol handle.
1-Benzylpiperidin-3-ol welcomes a variety of transformations. The hydroxyl group turns out to be a versatile spot for esterification or etherification, letting chemists attach larger pharmacophores or labels. Oxidation of that alcohol leads to the corresponding ketone, which occasionally serves as a valuable intermediate for further ring-opening or ring-expansion reactions. Halogenation, acylation, and N-debenzylation each have their roles, especially when labs need to build related scaffolds or explore structure-activity relationships (SAR) for new drug candidates. Introducing bulky groups at the three-position or on the benzyl moiety often fine-tunes lipophilicity or bioactivity, a tactic seen in medicinal chemistry campaigns where minor synthetic tweaks sometimes unlock major pharmacological shifts.
Across academic papers and supplier catalogs, 1-Benzylpiperidin-3-ol turns up under names like N-Benzyl-3-hydroxypiperidine and 3-Hydroxy-1-benzylpiperidine. Misspelled variants or inverted ring descriptors sometimes fill out older literature. A handful of product codes employ abbreviations such as 1-Benzyloxy-3-piperidinol, though this variant commonly refers to an ether derivative, reminding chemists to double-check source data. In regulatory filings and patents, the preferred name usually rests with IUPAC guidelines, which helps keep confusion low when tracing documentation or import-export papers.
No matter how familiar someone feels in the lab, handling chemicals like 1-Benzylpiperidin-3-ol calls for gloves, goggles, and solid ventilation. Direct contact can cause mild skin or eye irritation, though it ranks as less hazardous than more volatile amines or solvents. Accidental ingestion or inhalation brings a risk of dizziness or drowsiness, which stems from its CNS-related bioactivity. I’ve seen safety audits where teams sometimes neglect fume hoods on “quick” procedures—that’s a risk nobody should flirt with. Waste collection should avoid sinks, routing all liquids and contaminated disposables into labeled organic containers according to local regulations. Spills and leaks respond well to industrial absorbents and routine soap-and-water decontamination, minimizing residual hazards after cleanups.
Research teams in pharmacology and neurochemistry often use 1-Benzylpiperidin-3-ol as a precursor for analogs of psychoactive compounds. Its backbone fits nicely in screening libraries aiming to target dopamine, serotonin, or acetylcholine pathways. Small tweaks to the core skeleton mean labs can rapidly assess shifts in receptor selectivity or metabolic profile. Some groups probe it as a starting point for molecular probes or imaging agents, capitalizing on the ease of functionalizing the alcohol group or attaching radiolabels. The molecule also pops up in industrial R&D, where derivatives sometimes act as intermediates in the hunt for new agricultural or veterinary products.
Much of the present-day work involving 1-Benzylpiperidin-3-ol emerges from academic settings, where graduate students and postdocs chase new central nervous system agents or SAR studies. Industry has its eye on time- and cost-efficient syntheses, especially for late-stage drug candidates. Machine learning and computer-aided design now speed up the search for derivatives, crunching through countless permutations for binding energy or metabolic stability predictions. A trend I’ve noticed involves blending traditional synthetic chemistry with biocatalysis, particularly for greener alcohol formation at the three-position. Both start-ups and big pharma continue to invest in process optimization, using flow chemistry and telescoped purifications to reduce waste and streamline scale-up.
Although its structure and function seem unassuming, any compound with central nervous system activity earns a spot in toxicology screens. Rodent studies show dose-dependent neurological effects—drowsiness, mild tremors, and altered gait surface above certain thresholds. Short-term exposure carries low acute toxicity, yet repeated doses can produce behavioral shifts or minor hepatotoxicity according to some animal data. The hydroxyl group’s presence doesn’t offset all risks; metabolic pathways often yield active quinone or iminium intermediates, which labs need to consider during long-term exposure studies. There is no widespread industrial use, which limits large-scale human exposure data, but research work still aims to clarify both mutagenicity and reproductive toxicity, particularly if derivatives progress toward drug candidates.
Looking ahead, the compound’s future likely sits at the crossroads of medicinal chemistry demands and smarter synthesis practices. As drug discovery keeps scouring for selective CNS-targeting molecules, intermediates like 1-Benzylpiperidin-3-ol will hold their spot on synthetic routes and in fragment-based libraries. Advances in automated chemistry labs could further lower costs, allowing researchers to pump out derivative series with less manual labor or hazardous waste. If regulators push for stricter hazard assessment, more in vitro and in silico testing will probably fill current knowledge gaps in toxicity. Some see it forming the bedrock for new generations of neurotransmitter modulators, coupling old-school scaffold hopping with modern AI-driven SAR exploration. The piperidine ring has already shaped decades of pharma, and as labs keep tinkering with the basic framework, new opportunities for 1-Benzylpiperidin-3-ol will almost certainly open up.
Walk into any research lab these days and you'll spot a stack of containers lined up in the corner, each with a chemical name more complex than the last. Among them, 1-Benzylpiperidin-3-ol pops up. The folks in white coats know it by sight—a piperidine ring with a benzyl group and a hydroxyl hanging off to the side. It's not just sitting idle for the sake of chemistry trivia. Researchers keep reaching for this compound.
1-Benzylpiperidin-3-ol has grabbed attention in drug development. Medicinal chemists see promise thanks to its architecture. The piperidine core shows up in all sorts of prescription products, especially those affecting the nervous system. Tweak the chemical just a bit—add, drag, swap—and there's a chance for new molecules that can change how the body responds to disease. Sometimes new versions work as potential candidates for psychiatric medication, or act as reference standards, helping teams compare effects and side effects. Drug discovery rides on small shifts like these.
Back during some internship days at a university lab, I remember how often we scrambled for unique intermediates. 1-Benzylpiperidin-3-ol played the middleman. It serves as a building block to make other, even more complicated molecules. For every new project, people want “synthetic flexibility”—a kind of open road for making analogues. This compound offers that. There are also chemical suppliers who appreciate how researchers push the demand curve by developing new derivatives and related scaffolds.
While 1-Benzylpiperidin-3-ol attracts attention for good science, responsibility can't get lost in the shuffle. Some chemical cores linked to piperidines wind up in the wrong news headlines, drawing scrutiny from health authorities. Law enforcement and regulatory bodies keep a close eye on chemicals that could find their way into unregulated settings. Reliable suppliers restrict sales and ask for documentation from buyers for this very reason. Researchers learn pretty quickly which chemicals require extra care, and piperidine derivatives often appear on that learning list.
The scientific community keeps making progress in responsible management. Good lab practices now include routine chemical tracking, waste disposal according to local laws, and regular training on safety and legal requirements. Universities invest in chemical inventory systems to make tracking almost automatic. On the industry side, some suppliers have strict identity verification and only release shipments to accredited labs. These steps keep the wheels of progress turning without risking safety or reputation.
I’ve watched early-career researchers get frustrated by layers of paperwork or slow delivery times. It seems inconvenient, but these processes guard against a bigger headache down the road. Instead of racing to cut corners, the best teams focus on documentation and clear communication with all stakeholders. That keeps everyone safer—and helps promising compounds like 1-Benzylpiperidin-3-ol stay in the business of discovery, not distraction.
Every generation of chemist finds new uses for familiar cores. Fact is, 1-Benzylpiperidin-3-ol has earned a place on shelves because it fits into so many projects. It acts as a point of departure, a small leap to something much bigger. Managing it thoughtfully keeps doors open for research and innovation.
Curiosity leads people to all sorts of new compounds, and 1-Benzylpiperidin-3-Ol is one of those that pops up on forums and research papers. It does not have an established track record for medical use, so transparency matters when talking about what it does in the body and what can go wrong. My own interest in experimental substances comes from working alongside researchers investigating novel molecules after hours in the pharmacy. One clear thing: never brush off the risks that come from unclear safety profiles.
Only a handful of animal studies and user reports touch on this compound’s effects. A person experimenting with 1-Benzylpiperidin-3-Ol might feel restlessness, sleeplessness, rapid heartbeat, or dry mouth. Many piperidine derivatives—think of certain ADHD or antidepressant drugs—often amplify anxiety for sensitive folks, so stress levels may climb for some. Noticing muscle twitches, loss of appetite, or headaches? That does not always fade after a short while. Like with stimulants or psychoactives, the line between a pleasant effect and real distress runs thin, and doubling a dose by mistake raises the likelihood of serious trouble fast.
Long-term risks are an even bigger question mark. Some similar structures have caused bladder irritation, hypertension, and persistent sleep cycles problem in rats, and several studies I have read suggest nerve and liver sensitivity. Piperidine compounds can linger in the body and sometimes spur toxic reactions, especially for people with less efficient enzymes because of their genes. That’s a messy roll of the dice if you don’t know your body’s chemistry well.
Buying from unknown labs or international vendors is its own gamble. Purity matters—byproducts and leftover reagents from rushed syntheses can add nausea, chemical burns, or even seizures. During one review for a friend, I found a product labeled 1-Benzylpiperidin-3-Ol had three other compounds mixed in—not what you want near your central nervous system. Research chemicals do not always get tested before sale, so you might get a dose higher than expected.
Doctors and pharmacists rarely see this compound in their practice, but their experience helps spot warning signs early on. A well-trained toxicologist can interpret small warning flags in bloodwork or ECG results that the average person misses. Pharmacists pay attention to any history of heart arrhythmias, liver concerns, and psychiatric conditions, which play a big role in predicting side effects. If someone close to you starts describing agitation, confusion, or chest tightness after taking it, bringing in a professional right away can save a life. I have sat through cases where a bad batch led to emergency interventions—nobody expects bad luck, but it finds people ready or not.
Tighter regulation would help keep contaminated or mislabeled versions far from curious experimenters. Sharing results from preclinical toxicity screens as soon as they’re available makes a difference for everyone, not just scientists. People need decent access to healthcare without fear of stigma when things go sideways. For now, steering clear sounds boring, but from where I stand, guessing games about an unstudied compound’s toxicity are not worth the risk. Still, informed choices come from tough conversations, honest reporting, and keeping an eye out for real side effect patterns.
Anyone who’s spent time in a busy research space knows the importance of storing chemicals like 1-Benzylpiperidin-3-Ol with care. Years back, I remember the sharp odor drifting from a poorly capped vial in a shared chemical fridge. That experience never faded because folks took shortcuts on storage. Mistakes like that don’t just end with a foul smell—they can cost time, money, and safety.
1-Benzylpiperidin-3-Ol is an organic compound, best known in the lab for its role as an intermediate. It’s not enough to lump it in with “general organic chemicals.” Well-kept material lasts longer and stays reliable for research, which matters when you’re chasing consistent results. Typical chemical sense says moisture, oxygen, strong light, and heat all mess with sensitive compounds. With 1-Benzylpiperidin-3-Ol, keeping it dry and cool helps prevent breakdown and contamination.
Beyond its stability, storing volatile compounds calls for sealed containers. Glass vials with tightly fitting PTFE-lined caps do the trick—the standard plastic isn’t always up to the task, especially where solvents or slight acidity may come into play. Labels need to remain legible and detailed, or else future confusion steps in. Trust me, rummaging through a fridge packed with faded or missing labels creates headaches fast.
Safety data sheets wrap up most of the recommendations, but in busy work, folks skip reading them. Real talk: skin or eye exposure should always be avoided. Wear goggles and gloves, especially during transfers or weighing. Spills need cleanup with absorbent materials and a splash of patience. Don’t store organics near strong oxidizers or acids. If you store reagents in a fridge, slap a clear chemical warning outside—never use kitchen fridges for chemicals. I learned early not to trust "shared space" unless clearly marked.
Practical changes made a difference in my work. Labeled secondary containers (plastic boxes with a seal and a bold note) cut down cross-contamination. Consistent labeling won over fancy inventory software. Periodic checks and a quick whiff-test (before opening) flagged decomposing stocks. Compounds that turn yellow or opaque need to be pitched—they’re probably not safe or useful anymore.
Efficient chemical storage doesn’t just keep 1-Benzylpiperidin-3-Ol pure; it sets a tone. New students and old hands respect spaces where clear habits keep everyone safer. The real win: less waste, fewer near-misses, and more reliable results every time you reach for the right vial.
1-Benzylpiperidin-3-ol doesn’t sound familiar to most people, but it’s a molecule with ties to chemistry labs, research circles, and in some cases, the shadowy world of designer drugs. The challenge starts with one simple question: Can you legally buy or possess this substance? The answer isn’t straightforward, and anyone trying to make sense of it needs to look past rumors and focus on laws that actually exist.
This chemical doesn’t show up on official lists like the U.S. Controlled Substances Act, at least not directly. Lawmakers in America focus tightly on drugs known to carry risks or have a big potential for abuse. At the federal level, I couldn’t find 1-Benzylpiperidin-3-ol by name on Drug Enforcement Administration schedules. That might sound like a green light. The real story is a bit trickier. Regulations like the Federal Analogue Act allow the government to treat new substances like controlled drugs when their structure or intended use lines up with banned compounds. If someone sells or consumes this compound for recreational effects, they’ll likely land in legal trouble.
Scientists, law enforcement, and lawmakers often keep an eye on substances by comparing their structures to banned ones. 1-Benzylpiperidin-3-ol looks somewhat similar to compounds that pop up in designer stimulant markets. If a substance closely resembles amphetamine or other regulated stimulants, prosecutors might argue in court that it counts as an analogue—even if it doesn’t have a schedule. Nobody wants to risk a felony charge just because they didn’t look up chemical cousins first.
Laws shift across borders. Nations in the European Union, the UK, Canada, and Australia use their own systems for scheduling drugs and their analogues. Some European countries update controlled substance rolls or draw “generic” bans to keep up. Canada sometimes clamps down ahead of recreational use, while other countries take time to assess new risks. What’s legal to buy online in one country might get a person arrested at customs somewhere else. Even a trained chemist would double-check local law before shipping anything unusual across continents.
Ambiguity makes life harder for honest researchers, too. Labs and universities need to tread lightly, going through hoops like DEA licenses and substance reporting, even for chemicals not obviously controlled. The same rules protect public health, but delays and paperwork also slow down legitimate science work. Professionals often reach out to legal teams and regulatory agencies for written confirmation before handling gray-area compounds. Anecdotally, I’ve heard of researchers who change their project focus just to avoid these headaches—too risky, especially with limited university resources and funding on the line.
Lack of clear answers creates confusion for buyers, sellers, and medical teams who see patients affected by unknown chemicals. More transparent, regularly updated guidelines could help. Easy-to-search lists by country, backed up by clear government definitions, would save professionals and the public from guesswork. Open conversations between chemists, lawmakers, and health experts can help strike a real balance between public safety and legitimate science. Until those systems grow, caution rules the day. Anyone considering working with or possessing novel chemicals should get legal advice first—common sense can save careers and keep people out of court.
As folks poke around in advanced chemical research, especially those curious about central nervous system agents, compounds like 1-Benzylpiperidin-3-Ol start to show up. Some peer-reviewed papers look at new synthetic paths, potential pharmacological profiles or metabolic properties. Whenever a new structure comes into focus, people start asking: “Is there any safe or recommended dosage?” That question stands for a good reason — dose sets the balance between research progress and staying safe.
In labs, responsible chemists use extreme caution. While one can easily find suggested protocols for simple chemicals like ethanol or caffeine, the veil of mystery grows thick for newer or lesser-known molecules, especially those with psychoactive or unknown toxicological potential. There are few or no established, peer-reviewed, human dosing guidelines for 1-Benzylpiperidin-3-Ol. Reputable journals and chemical catalogues refer to it as a “research chemical,” mostly for reference samples, receptor mapping, or synthetic method evaluation.
On the bench, you won’t see 1-Benzylpiperidin-3-Ol next to acetaminophen or ibuprofen. This is because regulatory bodies — FDA, EMA, and their peers — have not evaluated it for drug use, setting any kind of clinical standard, or giving it approval as a treatment. Even animal studies are limited, with very little public or published work describing acute, repeat-dose, or chronic toxicity profiles. This leaves researchers with little guidance.
Dosing without this background can court disaster. Anybody considering unsupervised use — outside regulated, professional research — puts themselves at significant risk of off-target effects, unknown interactions, or organ damage. There’s also the ever-present risk of legal trouble, since many compounds with similar structures are controlled or restricted in different parts of the world under analog laws or designer drug statutes.
The ethical playbook suggests starting at the smallest measurable amount. If cell cultures or animal subjects get exposed, dosages often fall in the micromolar or milligram-per-kilogram range. This comes only after risk assessment, detailed review of structure-activity relationships, and comparison with similar compounds. Analytical chemists lean on mass spectrometry and chromatography to confirm purity, since any contaminant could complicate data or introduce new risks.
Any experiments involving living subjects call for a review board approval — sometimes labeled as Institutional Animal Care and Use Committee (IACUC) or Institutional Review Board (IRB). Data from chemical suppliers or academic partners usually describe the substance as “not for human consumption.” This is not empty legal jargon. Skipping preclinical studies or thinking that online anecdotes are enough is a shortcut with consequences. My own early lab training drilled this in through repeated safety talks and oversights. One senior researcher I knew stressed, “Our first mistake might be our last. Measure, record, question everything.”
Online forums and underground sources sometimes fill the information gap with speculation. Guidance pulled from one molecule rarely transfers neatly to another, especially with piperidine derivatives. One batch might not match another in strength or purity, making one person’s “safe dose” unsafe for the next. Without legit published human data, the only honest answer remains: nobody outside controlled laboratory settings should experiment or set casual dosing regimens.
The need for transparent, peer-reviewed science never fades. That means more studies, better toxicology data, and clarity about what these new chemical entities can do. Until research catches up, strict caution rules the day. That’s what experience and basic ethics call for — measure twice, experiment once, and avoid guesses or unchecked risks.
| Names | |
| Preferred IUPAC name | 3-(Phenylmethyl)piperidin-3-ol |
| Other names |
1-Benzyl-3-piperidinol 3-Hydroxy-1-benzylpiperidine |
| Pronunciation | /ˈwʌn ˈbɛnzɪl paɪˈpɛrɪdɪn θriː ɒl/ |
| Identifiers | |
| CAS Number | 5449-60-5 |
| 3D model (JSmol) | `3D model (JSmol) string` for **1-Benzylpiperidin-3-ol**: ``` COc1ccc(cc1)CN2CCC(CC2)O ``` This is the SMILES string for the compound, usable in JSmol for 3D visualization. |
| Beilstein Reference | 1206492 |
| ChEBI | CHEBI:19108 |
| ChEMBL | CHEMBL491283 |
| ChemSpider | 20375723 |
| DrugBank | DB08798 |
| ECHA InfoCard | 0564915d-2b68-45a9-a651-0b7d4e29c221 |
| EC Number | Emerging Chemical: Not assigned |
| Gmelin Reference | 114733 |
| KEGG | C15686 |
| MeSH | D065522 |
| PubChem CID | 107985 |
| RTECS number | TK4850000 |
| UNII | 1JZZ934XOD |
| UN number | UN3271 |
| CompTox Dashboard (EPA) | DTXSID60889536 |
| Properties | |
| Chemical formula | C12H17NO |
| Molar mass | 207.30 g/mol |
| Appearance | Colorless oil |
| Odor | Characteristic |
| Density | 1.08 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 1.95 |
| Vapor pressure | 0.000083 mmHg at 25°C |
| Acidity (pKa) | 15.06 |
| Basicity (pKb) | 4.11 |
| Magnetic susceptibility (χ) | -73.98×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.561 |
| Viscosity | 20.7 cP |
| Dipole moment | 2.48 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 395.9 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | N06BX19 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | `[H][C@@]1(CN(CC2=CC=CC=C2)CCN1)O` |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P280, P302+P352, P305+P351+P338, P312, P332+P313, P337+P313, P362+P364 |
| Flash point | 107.1±22.6 °C |
| Lethal dose or concentration | LD50 (rat, oral): 380 mg/kg |
| LD50 (median dose) | LD50 (median dose): 350 mg/kg (oral, rat) |
| PEL (Permissible) | Not established |
| REL (Recommended) | REL (Recommended): 10 mg/m³ |
| IDLH (Immediate danger) | IDLH not established |
| Related compounds | |
| Related compounds |
3-hydroxypiperidine 1-benzylpiperidine 1-benzyl-4-piperidone 3-piperidinol benzylamine |