People first took note of 1-Benzylpiperazine in the mid-1970s. At the time, research labs searched for new antidepressants and possible alternatives to amphetamines, eyeing this molecule for its known stimulant effects. Scientists realized that it boosted the release of dopamine and serotonin, producing a mental high not too different from what some partygoers chased. Chemists kept tweaking its structure, trying to balance therapeutic effects with safety. The chemical soon drifted out of labs and into the hands of those seeking a legal buzz, particularly in the early 2000s, when it surged in popularity across Europe, New Zealand, and parts of Asia as a ’party pill’ additive. Lawmakers scrambled to catch up, and the compound’s reputation fluctuated with every change in regulation.
1-Benzylpiperazine, or BZP, lands on shelves as a white to off-white powder or as pressed tablets—though powders show up more often with online suppliers. It falls under the synthetic stimulant crowd, often getting paired with other piperazine derivatives in so-called “legal highs.” Some folks in the chemical trade use BZP as an intermediate, building other complex organic molecules from its core. Its use in consumer products outside the party pill scene stays minimal, due to tight controls and its reputation for unpredictable side effects.
The structure sports a benzyl group attached to a six-membered piperazine ring. BZP clocks in with a molecular formula of C11H16N2 and a molar mass near 176.26 g/mol. The compound melts at around 196-198°C, and has moderate solubility in water but dissolves better in organic solvents like ethanol and acetone. BZP gives off a faint amine odor at higher concentrations. Its crystalline form arises when purified through slow solvent evaporation, and its stability stands up under room temperature, though sunlight speeds up degradation.
Packaging for BZP needs durable, clearly-labeled containers, usually with tamper-evident seals and printed batch numbers to trace origins and purity levels. Chemical suppliers have moved toward comprehensive safety data sheets, listing hazard codes (such as GHS/CLP) and recommended precautions. Regulatory compliance for transport involves warning labels for psychoactive substances and a clear indication if intended purely for research. Analytical grades, often 98% purity or above, make the cut for scientific use, not for recreational manufacture or sale.
Synthesizing BZP hinges on benzyl chloride’s alkylation of piperazine. You start by mixing benzyl chloride and piperazine in an organic solvent—like toluene or ethanol— and adding a base, often sodium carbonate, to soak up hydrochloric acid released during the reaction. After a few hours under reflux, the mixture cools, and the crude product precipitates out or is extracted with water. Filtration and recrystallization polish the yield, and chromatography mops up remaining contaminants. Some routes swap out benzyl chloride for benzyl bromide, or push the reaction with microwave heating, though traditional methods still dominate.
BZP’s structure invites all sorts of modification. Chemists add substituents to the aromatic ring or swap one piperazine nitrogen with other functional groups, changing activity and toxicity. Reductions can remove the benzyl group, while acylation or sulfonation introduces protections or solubility tweaks. In one well-trodden pathway, oxidation throws a carbonyl group onto the benzyl portion, making metabolites easier to detect in toxicology screens. BZP itself stands up to mild acids and bases but can hydrolyze slowly under harsh conditions, producing small amines and benzoic acid relatives upon breakdown.
1-Benzylpiperazine goes by a fistful of names. In scientific circles, it’s BZP or N-benzylpiperazine. Street lingo often shortens it to “piperazine” even though true piperazine lacks the benzyl group. Sometimes, internet vendors toss around product names like “Legal X” or “Party Pill Additive” to duck regulatory filters, muddying the naming waters. Other registry numbers—such as CAS 2759-28-6—help clarify which molecule’s on hand, cutting through slang and mislabeling.
Handling BZP in a lab means suiting up with gloves, goggles, and lab coats. Fumes can sting, so fume hoods make up standard equipment. Storage requires locked cabinets out of sunlight, kept cool and dry. Agencies like OSHA and European REACH stress preventing exposure by labeling all containers, training chemists, and tracking every gram moved or stored. Law enforcement in many countries treats BZP as a controlled substance, so legitimate research or trade demands locked inventory, detailed usage records, and tightly restricted access.
Most of the heat behind BZP comes from its time as a recreational drug, standing in as a stand-in for ecstasy (MDMA) when authorities cracked down on other club drugs. During its boom years, BZP filled energy drinks and pressed pills across Europe, Australia, and New Zealand. Flashing warning lights over side effects—panic, vomiting, seizures, even fatalities—pushed most countries to ban it outright, killing the “legal high” market. In research, BZP serves as a reference standard in forensic labs, helping analysts flag and confirm club drug seizures. Its stimulant profile drew some fleeting pharmaceutical interest, but the risks far outweighed any potential antidepressant or cognitive-enhancing uses.
Early pharmacology studies traced BZP’s fingerprints in the brain, especially its ability to spike neurotransmitter levels. Animal tests repeated patterns linked to amphetamines: hyperactivity, anxiety, and at higher doses, convulsions. Researchers also mapped how BZP metabolizes, noting both simple N-dealkylation and aromatic hydroxylation routes. Forensic toxicologists now routinely screen for BZP and metabolites in hair, urine, and blood of club scene detainees or overdose cases. Modified piperazines developed in modern labs either seek better safety profiles or serve as chemical probes to understand how stimulants tick at the synaptic level.
Much of the harm tied to BZP stems from its unpredictable dose-response. At low to medium doses, users report euphoria, alertness, and sometimes agitation. Taking more—especially with other substances like alcohol or MDMA—raises the risk of heart palpitations, high blood pressure, paranoia, and in some cases, seizures. Medical case reports link BZP to muscle breakdown (rhabdomyolysis), kidney failure, and rapid swings in body temperature. Rodent studies underline the risk for brain and liver damage, especially with repeat dosing. Regulators rely on these data to keep bans in place and to warn consumers about the real dangers beyond temporary “highs.”
The future for BZP looks closed on most commercial and consumer fronts. Legislation now blocks its route to the club scene in much of the world, and pharmaceutical companies turn away for good reason. Any research that survives circles around forensic detection, epidemiology, and mapping long-term sequelae in past users. Chemists who still work with BZP stick to developing safer analogues and robust detection methods. The story of BZP stands out as a lesson on how quickly designer drugs can move from lab curiosity to mainstream hazard—and how slow some systems move to rein them back in.
Some chemicals gain a reputation because researchers see promise in them, or because they pop up in everyday culture for reasons nobody wanted. 1-Benzylpiperazine, also called BZP, sits in a strange space between labs and late-night stories. Decades back, scientists tinkered with this molecule, looking for a safer version of antidepressants or drugs for the mind. Compared to most of the world’s medicine cabinets, BZP never found a rightful place. It doesn’t show up at the pharmacy. It hasn’t cured pain or lifted moods in ways doctors feel safe recommending.
Early studies explored BZP as a possible antidepressant and central nervous system stimulant. Researchers noticed the ways it made test subjects feel, seeing a little burst of energy, a spike of alertness—stuff that seemed handy at first. Problem is, side effects crept in. People got jittery. Sometimes, their hearts raced. Eventually, it became clear there’s little benefit but plenty of risk.
I’ve seen enough stories over the years where a lab hopeful turns out to be dangerous or gets abandoned because safer options exist. Doctors look for treatments that make life better, not ones that leave folks worse off. BZP fell by the wayside in medical circles, outpaced by other drugs that could do the job with fewer scary surprises.
That didn’t stop BZP from showing up elsewhere. For a while, especially in places like New Zealand in the early 2000s, it made the rounds in club scenes as a so-called “party pill.” Makers sold it as a legal alternative to MDMA (ecstasy), promising a cheap buzz without the risk of jail time. It sold in gas stations and corner shops, tucked into little packets with flashy logos. Kids bought it, sometimes thinking they were outsmarting the law.
Turns out, the safety pitch was shaky. Reports of rapid heartbeats, extreme anxiety, even seizures started rolling in. Emergency rooms worried about another epidemic. Governments saw the writing on the wall. One by one, countries banned it. Australia, most of Europe, the United States—all labeled BZP a controlled substance. These moves weren’t about moral authority as much as public health. No one wants to ride the ambulance for a night out at one of those clubs.
Some may ask: does BZP offer anything useful anymore? Short answer: just caution. Most chemists now consider it an example of what not to chase. It lingers in forensic labs, mostly for testing and tracking. Legal sanctions punish those who keep making or selling it. Medical studies still cite the risks—high blood pressure, agitation, even potential for addiction—without finding benefits worth the trouble.
If there’s a lesson, it’s about respect for what science and tough real-world experiences teach. BZP landed in a legal gray area for a while, but ended up right where so many risky fads go: off the shelves, out of the pharmacies, and mostly out of the headlines. People deserve better choices, safer nights out, and honest conversations about what these substances really do.
Walking into a pharmacy, most people wouldn’t expect to find something like 1-Benzylpiperazine, often shortened to BZP, on any shelf. It’s a compound that tells a tale twisting through parties, politics, and prison time. At its core, BZP is a synthetic stimulant. The story behind its legality shows how changing times push governments to keep up with shifting trends and public safety concerns.
BZP didn’t start out in nightclubs. Researchers developed it in the 1940s while searching for new drugs to treat infections. Later studies showed it had a stimulant effect that looked a lot like amphetamines. This made it less interesting to scientists, but by the early 2000s, manufacturers spotted its potential as a legal alternative to banned stimulants. Suddenly, party crowds began hearing about it as a component in “legal highs.”
Anyone trying to answer if BZP sits on the right side of the law faces a map splattered with red tape. In the United States, the federal government placed BZP on Schedule I of the Controlled Substances Act back in 2004. This means BZP gets grouped with drugs that authorities say carry a high risk of abuse with no accepted medical use. Local law enforcement has the authority to treat someone in possession of BZP the same way as someone caught with MDMA or methamphetamine. The U.K. took action in 2009 by adding BZP to its Class C drugs list. Australia, New Zealand, and many European countries have banned it as well. Lawmakers usually cite links to hospital admissions—ranging from agitation and seizures to more dire cases like heart issues—as a reason to clamp down.
There’s more than paperwork behind the ban. Reports show that emergency rooms witnessed more young people showing up after taking BZP, often under the impression it was “safe” since stores could sell it. In 2007, New Zealand researchers tracked young adults using BZP; four of every five users felt some unwanted effects. Even if serious cases stay rare, the confusion around the risks creates problems. I’ve seen friends shrug off synthetic drugs as harmless, only to end up sick, anxious, or needing help.
Banning a compound might keep it off shelves, but it doesn’t erase demand. Illicit labs can whip up knockoffs, drifting just outside legal definitions. Some of these replacements turn out even more dangerous, since few have been studied at all. Efforts to educate don’t always keep up with the pace of the black market. Most harm comes from not knowing what’s really in a pill or powder. Young people especially deserve straight answers about what they put in their bodies.
Drug bans send a message, but real change springs from talking honestly about why people turn toward stimulants like BZP in the first place. More support services, better drug checking, and up-to-date education tools can save lives. Governments have a responsibility to listen closely to researchers, emergency doctors, and—critically—those who use drugs. Real solutions mean paying attention to both the science and the social context.
1-Benzylpiperazine shows up in headlines mostly linked to synthetic party drugs. This compound, often known as BZP, feels like it popped out of nowhere a couple of decades back. In reality, folks working in labs created it for something completely different—potential use as an anti-depressant. It never caught on for that purpose because the side effects overshadowed potential benefits. Over time, it shifted into the illegal party scene. People use it hoping for a high that’s supposed to mirror amphetamines like MDMA or speed, but the ride takes unpredictable twists.
Some people have described a rush after taking BZP. There can be a burst of energy, a sense of euphoria, and sharpened alertness. Sounds appealing at first glance. Then the flip side hits. My conversations with users—especially from club and rave settings—paint a messy picture. Anxiety often creeps in, along with nausea, agitation, headaches, and in some cases, hallucinations. A night out that starts with excitement can wind up with panic attacks or heart palpitations. Some hospitals in New Zealand reported that the emergency room got packed with people coming in sweating, confused, and shaking, sometimes after just one dose.
BZP didn’t stay legal for long in most countries. Health agencies in Europe, New Zealand, and North America studied its impact and found patterns of harm—cardiac issues, seizures, and intense dehydration. I remember the 2005 New Zealand clampdown, which followed a spike in ER visits that doctors connected to BZP. Study after study showed more risk than reward—especially after mixing with alcohol or other stimulants. Reports suggest some users leaned into compulsive redosing, chasing the high, and ran headlong into sleep loss and near-psychotic episodes.
What sets BZP apart isn’t just its effects, but how little people know about it before trying. Amphetamines, for all their problems, have decades of research behind them. BZP entered streets with almost no proper testing on healthy humans. No doctor prescribes it. Most young adults I’ve met who experiment with club drugs have trouble even finding accurate dosing info beyond rumor and sketchy online posts. That lack of reliable data pushes risk higher.
The criminalization of BZP hasn’t erased demand, but it pushed supply underground. That makes tracing the source harder, reduces purity, and raises the odds of unexpected side ingredients. Harm reduction groups in places like New Zealand succeeded by running informational campaigns at music festivals. They set up free drug-checking booths and shared science-backed facts—no finger wagging, just honest talk. That helped some users make more informed choices and spot dangerous mixtures before swallowing something unknown.
Education, not just law enforcement, shapes outcome here. The most progress comes from pulling info out of the shadows. Doctors, paramedics, even night club workers, need real knowledge to spot and handle BZP casualties early. Those public conversations about risk can save lives. In my own experience listening to people who lived through BZP overdoses, the longing for empathy and honest answers runs deep. No one starts out hoping to end up in the ER. The more we put facts on the table, the better chance kids have at dodging mistakes that stick for a lifetime.
Many see 1-Benzylpiperazine (BZP) as just another recreational substance on the party scene. I remember conversations in college dorms, people suggesting it’s a "legal high" or an alternative to amphetamines. Rarely did anyone talk about what this chemical can actually do to a person over the long haul. Experiences from emergency rooms tell a harder story. You don’t need to see statistics to know things can get ugly fast.
BZP acts as a stimulant, ramping up everything that should be steady. Heart racing, jittery hands, dry mouth — these are warning signals. Doctors in New Zealand, where BZP once sat on gas station shelves, reported a steady trickle of young people with seizures, confusion, and erratic behavior after taking these pills. The risk multiplies if someone already has underlying heart issues. BZP pushes the heart, sometimes past its breaking point. British and Australian health studies showed plenty of cases where people ended up with chest pain, rapid heartbeats, or high blood pressure, all traced to BZP use.
Anxiety comes fast, often with paranoia tagging along. I’ve talked with people who wrestled with weeks of mood swings and insomnia after a single night out. Temporary euphoria feels like a tough trade when depression and agitation decide to hang around. British research documented panic attacks linked to BZP, especially when mixed with alcohol or other substances.
Then comes the risk of addiction. BZP works on the brain’s dopamine and serotonin systems, the same switchboard used by amphetamines. Cravings start showing up, sometimes even when someone swears they’re only dabbling. After a while, more is needed to get the same effect, and the hangover — both physical and emotional — deepens.
Heavy sweating, headaches, vomiting, and tremors leave a mark. Dehydration sets in quietly, which can lead to muscle cramps or kidney problems down the road. Doctors have seen patients come in for sudden seizures after taking a large dose, even if they felt fine moments before. In rare situations, hyperthermia — dangerously high body temperature — can take hold, especially in party environments where dancing and crowding bump up the heat.
Liver toxicity also raises its head. Reports from European hospitals traced noticeable increases in liver enzymes and rare cases of liver failure to regular use. No street pill comes labeled with purity or accurate dosage. Unknown contaminants make every tablet a gamble, and liver damage could be waiting in the wings.
Open conversation saves lives. People need real information from voices they can trust, not just scare tactics. Policy helps, but education matters more. Health professionals should listen without judgment, so fewer young people have to hide what really happens at parties. Schools and families should skip the preaching and explain what is truly at stake, drawing from medical evidence and lived stories.
Governments in some countries have already moved to restrict BZP. But bans don’t erase demand. Harm reduction works better than chasing every new designer pill off the shelf. Spaces with free water, drug-checking services, and onsite support during festivals make a big difference. Community groups can step up, sharing facts and safe practices, and steering friends away from risks before ambulances ever get called.
1-Benzylpiperazine, better known as BZP, enters the scene as a small white powder, often sold by the gram, sealed tight. These little bags travel fast—on campus, in city clubs, at after-hours parties, and sometimes through less supervised corners of the internet. Young adults bump into BZP as an alternative to substances like amphetamine, drawn by curiosity and the promise of a different kind of night.
The most common route to take BZP is swallowing a pill or capsule. Dealers and underground labs press the powder into pills, often mimicking legal supplements. Some crack open the capsule, chasing the powder with water. A few feel braver and snort it, hoping for a punchier effect, similar to how some use cocaine. Mixing BZP into a sweet drink isn't unheard of, but doses can be tough to measure this way, raising risks.
These patterns don’t show up in official guides—most people learn methods through word of mouth or online forums. I’ve seen college freshmen swap stories about which form hits harder. Social curiosity builds the knowledge base, not medical expertise.
Pills and capsules seem like the safest bet because the dose feels controlled and clear. Someone can pop a tablet, know mostly what to expect, and even split the dose with a friend. Snorting brings speedier effects, but that also means stronger risks—fast racing heartbeats and headaches often follow.
People look for a high without the heavy legal trouble, thinking BZP gives them a loophole away from criminal consequences or dangerous additives. Yet, the lack of legal oversight actually ramps up danger. With no quality standards and no clear dose labeling, people take their chances.
Health complications tell the story. Swallowing BZP leads to nausea, anxiety, sweating, and sometimes seizures. Snorting burns, creates sinus trouble, and raises the risk for faster, uncontrollable highs. Emergency room doctors now see more cases involving synthetic party drugs—identifying symptoms gets tricky when patients aren’t sure what or how much they took.
Sharing methods in peer groups also spreads misinformation. I once overheard a conversation at a local café, with one person insisting that sniffing would always deliver a better “roll.” Their audience listened, not realizing that rapid effects can mean rapid trouble, especially in bodies unprepared for stimulant surges.
People turn to BZP because they believe it sits in a legal gray zone, out of reach from conventional drug laws. Yet, governments worldwide now restrict it. This patchwork regulation fuels confusion. Honest, judgment-free education about effects and risks would help more than scare tactics or silence. Peer-led harm reduction projects show promise—these groups understand that people look for real advice, not just warnings.
Real-world harm drops when people access drug-checking services and learn to recognize higher-risk doses. Honest conversations beat whisper networks every time. Unregulated powders keep surfacing, and trying to ban information just pushes it deeper underground. What works is openness—directing people to accurate facts about BZP’s forms and effects, delivered by those they trust.
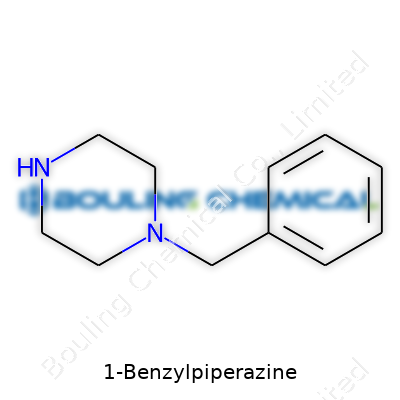
| Names | |
| Preferred IUPAC name | 1-benzylpiperazine |
| Other names |
BZP 1-Benzyl-1,4-diazacyclohexane Benzylpiperazine N-Benzylpiperazine |
| Pronunciation | /ˈwʌn ˈbɛn.zɪl paɪ.pəˈreɪ.zin/ |
| Identifiers | |
| CAS Number | 2759-28-6 |
| Beilstein Reference | 1209226 |
| ChEBI | CHEBI:14441 |
| ChEMBL | CHEMBL1477 |
| ChemSpider | 86515 |
| DrugBank | DB00877 |
| ECHA InfoCard | echa.europa.eu/infocard/100.046.293 |
| EC Number | 211-585-5 |
| Gmelin Reference | 69347 |
| KEGG | C10941 |
| MeSH | D010713 |
| PubChem CID | 8889 |
| RTECS number | UX0955000 |
| UNII | XY7V5M18J7 |
| UN number | UN2578 |
| Properties | |
| Chemical formula | C11H16N2 |
| Molar mass | 195.28 g/mol |
| Appearance | White solid |
| Odor | Odorless |
| Density | 1.03 g/cm3 |
| Solubility in water | soluble |
| log P | 1.89 |
| Vapor pressure | 0.0513 mmHg (at 25 °C) |
| Acidity (pKa) | 8.8 |
| Basicity (pKb) | 4.82 |
| Magnetic susceptibility (χ) | -68.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.569 |
| Viscosity | Viscous oil |
| Dipole moment | 3.03 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 299.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -4507.7 kJ/mol |
| Pharmacology | |
| ATC code | N06AX21 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Danger |
| Hazard statements | H302, H312, H332 |
| Precautionary statements | P261, P264, P271, P272, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362+P364 |
| NFPA 704 (fire diamond) | 2-2-0 |
| Flash point | 77.5 °C |
| Autoignition temperature | 310 °C |
| Explosive limits | Lower: 1.4% Upper: 6.9% |
| Lethal dose or concentration | LD50 (oral, rat): 657 mg/kg |
| LD50 (median dose) | > 460 mg/kg (rat, oral) |
| NIOSH | RN 2759 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 1-Benzylpiperazine: Not established |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Trifluoromethylphenylpiperazine Methoxyphenylpiperazine Dibenzylpiperazine mCPP MeOPP TFMPP BZP |