Chemistry moves forward with keen observation and a bit of boldness. Decades back, organic chemists exploring new synthetic routes in the mid-20th century landed on piperidine derivatives with growing interest. The path to 1-Benzyl-N-Phenylpiperidin-4-Amine began with curiosity about the reactivity of aromatic amines fused with piperidine rings. Early academic and patent literature from the 1960s spotlights growing attention toward this family, especially as researchers sought to bridge aromatic systems and secondary amines for pharmaceutical and industrial potential. Every experiment built more trust in how this structure could impact fields from drug synthesis to material science. The structure’s subtle tweaks over the years have shown how a single benzyl group or a specific position on the ring can dramatically shift physical and chemical profiles.
1-Benzyl-N-Phenylpiperidin-4-amine looks simple at first glance: a piperidine ring, a benzyl group at the first position, and a phenyl group tagging the nitrogen. Beneath this lies a world of function. Many labs recognize this compound as a core building block for custom ligands, pharmaceutical intermediates, and specialty chemicals. While not a household reagent, its ability to act as a skeleton for bioactive molecules gives it rare value. Demand for this compound often reflects trends in drug discovery and exploratory synthesis, echoing what researchers are chasing worldwide.
The compound presents as a solid or crystalline powder at room temperature, depending on purity and specific form. Its color may range from off-white to light beige. Odor tends to be faint, but there's a signature amine scent on close contact. Solubility falls on the side of organic solvents, with good dissolution in ethanol, methanol, chloroform, and DMSO, while showing poor mixing with water. Its molecular weight hovers around 292.4 g/mol. Melting point data varies by supplier, often landing between 70–90°C. Stability remains reliable under dry, cool storage—moist air and high temperature, though, can coax unwanted degradation.
Chemists and procurement specialists expect clarity when ordering this compound. Reputable manufacturers label with precise purity—usually above 98% by HPLC or NMR—along with batch number, assay results, and shelf-life guidance. Storage instructions call for tightly sealed containers, out of direct sunlight, at temperatures below 8°C. Labels clearly mark hazard designations and recommended personal protective equipment. Transport manifests match international standards, ensuring safety in handling, from warehouse to laboratory bench.
Classic synthesis builds 1-Benzyl-N-Phenylpiperidin-4-Amine through reductive amination or nucleophilic substitution. One robust approach couples benzyl chloride with N-phenylpiperidin-4-amine under alkaline conditions. Catalytic hydrogenation over palladium on carbon sometimes finishes the job, reducing any imine intermediates. Reaction workups demand careful washing and recrystallization, as impurities linger in amine preparations more than in neutral compounds. Proper drying rounds out the workflow, locking in the desired physical form without residual solvents. A watchful eye through TLC or LC-MS at each step saves headaches downstream.
A chemist worth their salt recognizes several opportunities for modification. The secondary amine group opens up acylation, alkylation, or even carbamate formation, useful for tuning pharmacokinetics. The aromatic rings welcome substitution—halogenation, nitration, or oxidative coupling—each shift writing a new chapter for downstream products. Radical or transition metal-catalyzed transformations allow custom derivatives for SAR studies in medicinal chemistry. Whether working on antipsychotic analogs, CNS penetrants, or agrochemical prototypes, small changes ripple through a whole project.
Like any workhorse compound, naming varies across suppliers and research papers. Some catalogs list it as 4-(Benzylamino)-1-phenylpiperidine, while custom manufacturers refer to it by company product number or code. Common abbreviations include BPP-amine, Benzyl-N-phenyl-4-piperidinamine, or even truncated as BNPP4A in lab notes. This mix of nomenclature reflects its broad reach in both academic and industrial applications.
Working with aromatic amines and piperidine derivatives, respect for safety beats any shortcut. Gloves, goggles, and lab coats form non-negotiable gear. Inhalation or skin contact risks irritation or harmful effects, so strong ventilation matters. Spills get cleaned with adsorbent pads, not just paper towels. Waste streams merge with other amine-rich residues and follow hazardous disposal protocol. Every chemical storage area should post clear signage for this compound, with emergency eyewash and showers within fast reach. Following OSHA, REACH, and local guidelines keeps teams out of trouble and research timelines on track.
The versatility of this compound catches the eye in more than just pharmaceutical circles. Medicinal chemists chase novel painkillers and CNS agents, leveraging the molecule’s ability to mimic known bioactive scaffolds. Polymer chemists harness it for functional materials requiring tuned flexibility and bonding. Agrochemists use derivatives in exploring selective pesticides or plant protectants. Each domain pulls from the same base, but the end goals split across fields as wide as human health, manufacturing, and food security.
Every research-driven company has a handful of piperidine derivatives in the pipeline for early-stage screening. 1-Benzyl-N-Phenylpiperidin-4-Amine fits squarely as a structural motif in proposed serotonin and dopamine analogs for psychiatric research. Patent filings over the last ten years reveal renewed interest in its core for treating pain, neurodegeneration, and even rare metabolic disorders. Its modular nature keeps it relevant as libraries are built, broken down, and rebuilt to chase elusive biological targets.
No chemist dismisses toxicity studies as box-ticking. Many aromatic amines trigger red flags due to genotoxic risk, and piperidine backbones rarely escape scrutiny. Preclinical studies on related analogs report mild to moderate acute toxicity—mainly CNS depression and metabolic alteration in rodent models—though most results require side-by-side comparisons. Chronic toxicity often relates to repeated exposure, underlining the need for strong handling practices and accurate labeling in labs. Ongoing research around metabolic byproducts seeks to explain long-term risks and guide rational design for safer derivatives.
As drug hunters expand the search for novel neuroactive scaffolds, this compound maintains a strong foothold. Computational chemistry and machine learning in structure-based drug design only boost stocks for modular intermediates like 1-Benzyl-N-Phenylpiperidin-4-Amine. Improvements in green synthesis and purification extend its sustainability profile, answering new environmental standards. Regulations grow tighter each year, so more attention falls on tracking, usage logs, and digital inventory. Research labs and manufacturers that keep pace with these trends transform a classic structure into tomorrow’s innovation, with potential spanning therapies, smart materials, and beyond.
Anyone who’s spent time in a chemistry classroom or poked around drug design knows those long chemical names mean a lot more than meets the eye. With 1-Benzyl-N-Phenylpiperidin-4-Amine, the name spells out who’s bonded to whom and where the pieces meet. The backbone here is a piperidine ring, which sits as a classic six-membered saturated ring, with five carbons and a nitrogen. Piperidine forms the core for plenty of medicines and research chemicals, not just because it’s easy to build on but because its shape can mimic biological molecules in the body.
Each word in the name reveals a fragment of the puzzle. “Benzyl” tells us there’s a CH2 plus phenyl group attached somewhere — in this case, it attaches to the piperidine’s nitrogen atom. The “N-Phenyl” portion says that a phenyl group connects directly to the nitrogen too, but this nitrogen sits at the fourth carbon position on the piperidine ring, making it the “4-amine” portion of the compound. Anyone drawing the structure would sketch a hexagon with a nitrogen atom, tag on a phenyl ring via a CH2 group at the ring’s nitrogen, and another phenyl group on the nitrogen of an amine linked at the fourth carbon.
Every detail in this name influences how the molecule might behave and interact. Medicinal chemists lean hard on these patterns for predicting binding to biological targets. The benzyl bit adds flexibility, helping it glue onto aromatic binding pockets in proteins. Phenyl rings, with their flat, stable arrangement, often slot neatly into enzyme crevices, much like fitting puzzle pieces together. Each ring and bond tweaks the molecule’s electronic and 3D makeup, shaping drug-like behavior and solubility.
Scientists and drug researchers don’t just memorize names for fun. Powerful psychoactive drugs — take methylphenidate or fentanyl — share similar piperidine cores paired with different attachments. Tweaking one group can mean the difference between a helpful medication and something far more dangerous. Recognizing a benzyl or phenyl attachment arms chemists with clues about metabolism, receptor activity, and legal regulation.
In research, knowing precise chemical structures allows labs to synthesize substances more safely and predict unwanted side effects. Mistakes in drawing or understanding molecules can lead to misidentified samples and even harmful health outcomes. My own lab work showed the risks of confusion firsthand: mixing up where a benzyl group connects could lead to inconsistent results, wasted resources, or real safety hazards for anyone handling the substances.
Attention to detail at the structural level serves patients, scientists, and society alike. Structural transparency in chemical reporting helps regulators keep illicit substances off the street and supports the safe rollout of new medications. Open databases, such as PubChem, give researchers verified diagrams and data, speeding along both discovery and public safety.
For students or professionals, focusing on structure isn’t about rote memorization. It means applying a detective’s eye, looking for patterns, and thinking through how one tweak changes everything. Teachers can make all the difference by linking abstract structure concepts to real-world applications, from drug development to toxicity prediction. Hands-on modeling, careful diagram reading, and lots of practice build true chemical literacy — the kind that solves problems and saves lives.
1-Benzyl-N-Phenylpiperidin-4-Amine doesn’t spark recognition for most people. For those working in medicinal chemistry, analytical labs, or chemical research, this compound pops up more often than expected. Let’s shine a light on what it actually does and why it keeps finding a spot on procurement lists and grant proposals.
Take one look at ongoing drug discovery projects, and you’ll see how certain chemical structures tend to stick around. The piperidine core found in 1-Benzyl-N-Phenylpiperidin-4-Amine shows up in medicines ranging from antihistamines to antipsychotics. Medicinal chemists latch onto this building block when looking for fresh lead compounds. They know that tweaked versions of piperidine derivatives sometimes help tackle bacteria that have sidestepped older treatments. Piperidine derivatives even show potential as pain relievers, especially when opioid addiction draws concern. This connection between 1-Benzyl-N-Phenylpiperidin-4-Amine and pharmaceutical progress signals its importance far beyond its name or shelf label.
Synthetic chemistry, the craft that builds molecules one step at a time, leans heavily on “scaffold” compounds. 1-Benzyl-N-Phenylpiperidin-4-Amine fits that role. Its structure, lined with both aromatic and amine features, offers chemists options for making more complex molecules. In graduate research projects, someone in the lab gets tasked with making new derivatives almost every semester. The goal isn’t always a blockbuster drug; sometimes, teams want a stable reference point for experiments measuring reaction speed, stereochemistry, or unusual chemical bonds.
Law enforcement and medical labs face growing challenges in drug identification. 1-Benzyl-N-Phenylpiperidin-4-Amine has shown up as a reference standard in high-performance liquid chromatography (HPLC) and mass spectrometry analyses. Testing kits calibrated with such chemicals help sort out illicit substances or track new psychoactive compounds on the market. Quality control counts for everything in these settings. One misidentified compound can sink an investigation or lead to wrong clinical advice.
Industry rarely wastes time on chemicals that don’t offer clear value. That said, specialty chemical suppliers stock 1-Benzyl-N-Phenylpiperidin-4-Amine because research institutions and pharmaceutical companies keep placing orders. These companies don’t just value it for what it can provide today; they see it as a stepping stone to something bigger. Not every project pans out, but the presence of this compound in a catalog means there’s continued hope it might help form a key intermediate for tomorrow’s drug candidate.
Of course, every chemical with pharmacological relevance brings questions of regulatory oversight and safety. Certain countries keep tabs on piperidine derivatives to prevent unsafe use. Rigorous safety protocols belong in any setting where these compounds get handled. Researchers wear gloves, work under fume hoods, and track every gram. Long before anything hits clinical trials, careful toxicology studies help weed out dangerous candidates.
If there’s one lesson, it’s that scientific value ties to the people behind the work, pushing boundaries with old and new molecules. Supporting sound research and investing in safer labs helps get the best out of compounds like 1-Benzyl-N-Phenylpiperidin-4-Amine, while staying ahead of misuse. With so much potential left in chemical research, those careful steps will keep driving discovery and solutions when they’re needed most.
1-Benzyl-N-Phenylpiperidin-4-Amine comes with a chemical structure that can pose risks in the form of toxicity and sensitivity once it hits air or skin. Small molecules like this often find their way through gloves that seem fine for ordinary work. The memory of a laboratory accident years ago, where a seemingly harmless organic compound led to burns, stays with scientists long after the event passes. This kind of experience teaches a respect for the unexpected reactions from such specialized chemicals.
Splash goggles do more than just check a box—they keep eye accidents from ending a week in agony. Gloves need more thought. Nitrile gloves usually hold up longer, but only if the chemical compatibility charts back it up. Lab coats always serve a real purpose, not just for photos. Airflow matters too; closed systems, fume hoods, or even well-maintained extraction units keep dangerous vapors away from lungs. Many folks have rushed through a procedure with the hood sash too high and paid the price, wheezing and watery-eyed. Stay behind the sash and keep it low.
Organics like 1-Benzyl-N-Phenylpiperidin-4-Amine need dry, cool, and dark storage. Damp benches or shelving ruins stability fast. Everyone in chemistry remembers at least one spill from a shelf stacked too high with bottles balancing like dominoes. Proper labeling saves time and reduces confusion; mix-ups with active pharmaceuticals or lab standards cause more headaches than the label takes to print. Using a tight-lidded container provides added security against accidental release or exposure.
No training truly prepares you for the first chemical spill, especially if the cleanup kit proves empty or expired. Build habits for good housekeeping. Know where the neutralizing agents sit—verifying them every month keeps confidence high. A spill never looks textbook, but the steps remain simple: evacuate, ventilate, and clean only with gloves and eye protection in place. Calling for help rarely feels heroic, but pride heals faster than a chemical burn or severe inhalation.
There’s no shortcut around disposal laws. Accumulating waste in sealed, clearly labeled containers avoids costly mistakes. Years ago, a nearby lab lost thousands in fines for improper labeling and mixing of hazardous waste. Local environmental departments outline waste codes for compounds with nitrogen and benzyl derivatives. Never let convenience trump compliance, since the real cost hits downstream: pollution, fines, or injury to waste facility workers.
Standard operating procedures make bad surprises less likely. Regular safety briefings, whether for new hires or seasoned chemists, force everyone to confront blind spots. Storytelling in training lifts it above rote learning, swapping dull slides for real mistakes and fixes. Near-misses deserve the same level of reporting as injuries since they often highlight a gap that could widen without action. With every form filled out, the next person facing the same scenario adapts the lesson instead of repeating the error. That’s a win for everyone around the bench and beyond.
Not every chemical compound lives in the same market. Research chemicals, especially those closely related to substances with known psychoactive effects, invite more skepticism and regulation than simple industrial solvents or common reagents. 1-Benzyl-N-Phenylpiperidin-4-Amine doesn’t pop up readily through most standard suppliers. Much of that comes down to its close chemical relationship with pharmaceuticals, or with compounds that don’t necessarily get used for benign purposes.
Laws guiding the sale of piperidine derivatives have tightened. A major reason comes from the misuse of similar compounds in the production of designer drugs. Regulatory bodies pay close attention to structural analogues of controlled substances. Online sources selling such chemicals frequently operate in legal gray zones or outside established regulations. Before even thinking about a purchase, every buyer should seriously examine the legal status of the compound in their country. Ignoring this step risks more than just a failed project—legal consequences can get severe, particularly around chemicals that cross into pharmaceutical or drug precursor territory.
Reputable chemical vendors ask for documentation: Institutional connections, end-use declarations, and valid research purposes. This isn’t about red tape for its own sake. Legal compliance keeps real research possible. Skirting these requirements puts both researchers and vendors in jeopardy, eroding legitimate access over time. Reputable labs recognize that documentation keeps everyone above board.
Chemical purity speaks louder than labels printed on a bottle. With regulated or obscure compounds, vendors often claim impressive numbers—98%, 99%—but fail to back those claims with hard data. That purity means little without third-party verification or proper documentation (like certificates of analysis with spectra or chromatograms). Purity impacts research outcomes. An impurity at 1% might sound small, but synthetic chemistry doesn’t forgive small details. Side products or trace contaminants distort reactions, poison catalytic steps, or create false signals in analytical work.
In my own work, I’ve seen frustration mount when an experiment fails from hidden contaminants. That frustration multiplies with rare or poorly controlled chemicals. Direct purchase of obscure compounds from “gray market” sources often ends with products that don’t match the label. Samples might contain unknown byproducts or even be substituted entirely. For reputable vendors, third-party analytical data is non-negotiable. Researchers also protect themselves by running independent checks using NMR, MS, or HPLC—even with documentation supplied. Researchers in pharmaceutical labs or university settings know the value of those chromatograms. They keep projects moving and reputations clean.
Some believe the only barrier is money or persistence. That misreads the deeper challenge. Sourcing research-grade chemicals such as 1-Benzyl-N-Phenylpiperidin-4-Amine means balancing curiosity with ethics, and speculation with safety. No research should hurry down paths that cross legal or ethical boundaries. Partnering with trusted suppliers, demanding analytical proofs, and sharing best practices within research communities strengthens the system. Regulatory clarity helps, too. Continuous advocacy for balanced regulation—neither draconian nor lax—protects research interests while discouraging misuse.
Scientists rely on trust. The chemicals in the cupboard shape not just projects, but careers and lives. Respect for that trust keeps research alive, even as the landscape changes.
Chemicals like 1-Benzyl-N-Phenylpiperidin-4-Amine don’t come with easy margins for error. From my years handling reagents in university labs and biotech spaces, it’s clear that the wrong temperature or a careless seal can ruin a batch—sometimes before you even know anything’s wrong. Mishaps don’t just cost money; in some cases, they threaten safety or research outcomes. For this particular compound, following smart storage guidelines helps avoid contamination, unwanted reactions, or early degradation.
The majority of sensitive organic amines demand cool, dry storage. Experience on research teams and with standard operating procedures backs up what most chemical catalogs advise: keep 1-Benzyl-N-Phenylpiperidin-4-Amine between 2°C and 8°C. I always use chemical fridges with temperature monitoring, steering clear of the common freezer used for enzymes and samples—condensation issues can sneak in when reagent bottles get moved around. A stable, dark place means less UV degradation or accelerated breakdown. Direct sunlight or hot warehouse shelves have a real impact. Recent studies point out that even a few days of warm storage can drop purity or cause color change.
Damp environments spell trouble for many substituted piperidines. Moisture can trigger hydrolysis or other side reactions, which might not show up until a failed experiment or inconsistent assay results. In my own experience, even humidity spikes in the lab—like after the air conditioning goes off—can gunk up screw-cap vials or leave residues along bottle rims. Desiccators work as insurance, especially if the chemical sits out between uses.
As for air, it’s not just about oxidation from oxygen but contamination from dust or volatile compounds in the lab. Tightly sealed containers, preferably amber glass or another airtight vessel, block both air and light. Single-use aliquots cut down on repeated opening. I’ve watched teams lose weeks of work because a “just one more use” approach let ambient air in, especially in rooms with heavy solvent use or regular traffic.
Mislabeling or storing incompatible chemicals together invites hazard. Years in chemical stockrooms taught me to spot the danger of putting amines near strong acids, oxidizers, or reactive metals. 1-Benzyl-N-Phenylpiperidin-4-Amine should stay well away from such substances. Color-coded tape or detailed logbooks help—if the label peels off, everything stops until it’s resolved.
Some recurring problems come down to hurrying or missed details. Investing in reliable refrigeration, dehumidifiers, and strong-sealing containers pays off over endless replacements or failed projects. Routine inventory audits help, especially in shared labs where supply and tracking break down easily. Digital tracking can flag improper temperature swings or missed supply dates. Regular staff training gives everyone a sense of ownership—a shelf or drawer might seem trivial until a single spill costs thousands.
For anyone with less experience, starting with supplier recommendations and peer-reviewed articles gives a good baseline. Even veteran chemists benefit from refreshers. Small steps—checking seals after every use, logging temperature daily, keeping backup labels close—build habits that serve safety and shield investments in lab resources and research.
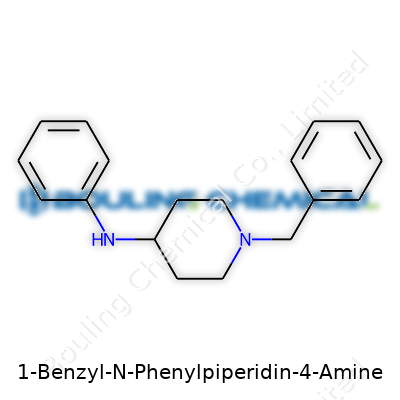
| Names | |
| Preferred IUPAC name | N-Phenyl-1-benzylpiperidin-4-amine |
| Other names |
4-Anilino-1-benzylpiperidine N-Phenyl-1-benzyl-4-aminopiperidine |
| Pronunciation | /ˈwʌn ˈbɛn.zɪl ɛn ˈfiː.nɪl paɪˈpɛr.ɪ.dɪn fɔːr əˈmiːn/ |
| Identifiers | |
| CAS Number | 137391-77-2 |
| 3D model (JSmol) | `CCCC1(CCN(C1)N(C2=CC=CC=C2)C3=CC=CC=C3)` |
| Beilstein Reference | 3119988 |
| ChEBI | CHEBI:76205 |
| ChEMBL | CHEMBL2103883 |
| ChemSpider | 159159 |
| DrugBank | DB08914 |
| ECHA InfoCard | ECHA InfoCard: 100.265.471 |
| EC Number | EC 620-352-5 |
| Gmelin Reference | 673509 |
| KEGG | C11406 |
| MeSH | D03.438.505.680.180 |
| PubChem CID | 11442752 |
| RTECS number | UG8753000 |
| UNII | 0I85P36B2N |
| UN number | UN3276 |
| CompTox Dashboard (EPA) | 6BJK5Q840L |
| Properties | |
| Chemical formula | C18H22N2 |
| Molar mass | 312.45 g/mol |
| Appearance | White solid |
| Odor | Odorless |
| Density | 1.1 g/cm³ |
| Solubility in water | Insoluble |
| log P | 2.96 |
| Vapor pressure | 0.0000227 mmHg at 25°C |
| Acidity (pKa) | pKa = 9.49 |
| Basicity (pKb) | 3.86 |
| Magnetic susceptibility (χ) | -74.72×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.609 |
| Viscosity | Viscous liquid |
| Dipole moment | 4.23 Debye |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 373.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -36.3 kJ/mol |
| Pharmacology | |
| ATC code | N06AX25 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07, GHS08 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P272, P302+P352, P312, P321, P363, P501 |
| Flash point | > 110 °C |
| LD50 (median dose) | LD50 (median dose): 63 mg/kg (rat, oral) |
| NIOSH | DJ2U06FZ9J |
| PEL (Permissible) | Not established |
| REL (Recommended) | 200-500 mg |
| Related compounds | |
| Related compounds |
Benzylpiperidine N-Phenylpiperidin-4-amine 4-Phenylpiperidine Benzylamine Piperidine |