Back in the 1970s, chemists in pharmaceutical research began working with piperazine derivatives, keen to uncover new compounds with psychoactive or therapeutic effects. One offshoot from that era—1-Benzoylpiperazine (BZP)—soon got attention for its capacity to mimic certain stimulant properties. Over the next decades, research crept along, often lagging behind newer molecular innovations. As countries updated drug laws and designer substances entered underground markets, BZP shifted in reputation from a possible medicine to a so-called party drug or research chemical. This checkered history made it clear that a deeper, more precise understanding of piperazine compounds was sorely needed—not just for law enforcement, but for anyone interested in chemistry, toxicology, or pharmaceutical development.
1-Benzoylpiperazine Hydrochloride forms a key link between the chemistry bench and practical research needs. It’s a synthetic compound, marked by a piperazine ring joined to a benzoyl group, with hydrochloride salt enhancing its solubility and stability. Unlike more famous family members like BZP, this derivative isn’t as widely publicized, but shows up in research catalogs, chemical supply inventories, and analytical labs worldwide.
This compound usually appears as a fine white or off-white crystalline powder. It dissolves easily in water and polar solvents due to the hydrochloride salt form, making it workable for lab applications. The benzoyl group attached to the piperazine ring adds rigidity and tweaks chemical reactivity. Its melting point sits in the moderate range, often between 180 and 200 °C, indicating decent thermal stability. With a molecular weight under 250 g/mol and a formula of C11H14N2O•HCl, it falls well within the handling parameters for mid-range organic molecules in research settings. Instant recognition of such properties can help chemists make snap judgments about solvent choices or reaction atmospheres, avoiding wasted time and costly failures.
High-purity 1-Benzoylpiperazine HCl must meet specifications demanded by scientific work: purity above 98%, well-labeled batch numbers, and detailed certificates of analysis. Authentic suppliers publish full spectra—NMR, IR, and MS—for verification. A good supplier will ship the powder in moisture-resistant, light-proof containers and attach chemical safety data sheets with hazard warnings in plain language. Clear labeling helps researchers avoid nasty surprises in storage rooms and keeps compliance officers off their backs.
Synthesis often kicks off with piperazine as a base scaffold. A benzoyl chloride is reacted with piperazine under controlled conditions in a solvent like dichloromethane, with a base such as triethylamine soaking up byproduct hydrochloric acid. After the reaction, purification follows using crystallization or column chromatography. Once the benzoylpiperazine core is isolated, adding hydrochloric acid either as a gas or in solution turns the compound into its salt form, producing the stable, manageable HCl variant. Routinely, experienced chemists keep glassware dry, time the addition of reagents, and monitor reaction temperatures to squeeze out consistent yield and cleaner product.
Chemists love to tweak piperazine rings. Substitution on the nitrogen atoms, introduction of different acyl groups, or even swapping the aromatic ring can tune the biological or physical profile of the molecule. BZP’s benzoyl ring can undergo halogenation or nitro addition, creating analogs for screening new psychoactive effects or refining receptor affinity. That sort of work lays the groundwork for drug discovery, but not every change produces a practical or safe compound. Some modifications dial down activity, others open up untested toxicities, so careful, stepwise development and monitoring become absolutely necessary.
Across literature and chemical catalogs, the compound pops up as N-Benzoylpiperazine hydrochloride, BZP HCl, or simply 1-Benzoylpiperazine hydrochloride. Synonyms serve a practical purpose when searching reference materials, patent filings, or online databases. Ensuring all names connect back to the same molecular structure cuts through confusion and prevents mix-ups in procurement or data analysis.
Laboratory personnel working with this chemical follow strict operational routines: gloves, goggles, lab coats, and well-ventilated fume hoods. Accidental spills get contained with absorbent materials and cleaned with dilute sodium bicarbonate. Heat sources stay off unless reactions specifically demand them. In my own lab, Material Safety Data Sheets never gather dust—they serve as a first line of defense against accidental exposures. Real-world mishaps aren’t rare: a single oversight, like failing to label a flask, can lead to skin or eye exposure, which in turn means lost time, higher costs, and medical interventions. Regular training on chemical hazards and rapid response procedures keeps more serious accidents at bay.
1-Benzoylpiperazine derivatives touch several sectors. Pharmaceutical teams occasionally screen them for nervous system activity, aiming to uncover novel therapies for disorders like anxiety or depression. Analytical chemists employ these molecules as reference standards when testing street samples or probing biochemical pathways. In industrial settings, synthetic intermediates streamline processes involving larger heterocyclic molecules for agrochemical or dye manufacturing. Experience in the lab shows that wide-ranging chemical utility always brings both opportunity and responsibility to ensure safety and compliance.
Academic groups dig into structure-activity relationships, searching for tweaks to improve efficacy or reduce toxicity. Medicinal chemists target subtle changes in the benzoyl or piperazine backbone to balance metabolic stability with receptor selectivity. My experience working alongside these teams has shown that even minor molecular dial-ins can lead to months of exploratory work. In the last decade, research trends have migrated toward computational modeling and in vitro testing to speed up screening and filter candidates before animal trials. Funding bodies, challenged by evolving regulatory landscapes, now demand clear justifications and risk assessments, making strong documentation and open data sharing valuable currency in R&D programs.
Toxicologists track how these compounds interact with human and animal models, focusing on neurochemical disruption, organ effects, and metabolic breakdown products. A history of abuse by recreational users brought additional urgency to these studies. Results often reveal risks of overstimulation, cardiovascular stress, and unpredictable interaction with other substances. Lab-based animal testing, coupled with modern cell culture models, continues to unravel exact pathways leading to adverse outcomes. Real-world poison center data highlights that even structurally related analogs can behave quite differently, so ongoing vigilance stays central to responsible handling.
As medical chemistry evolves, the piperazine core keeps earning fresh attention from those building out new therapeutic scaffolds. The challenge always lies in separating promising bioactivity from unacceptable side effects. AI-driven drug design, automation in reaction setup, and comprehensive toxicological screening will keep pushing these compounds toward safer, more precisely targeted outcomes. Regulatory changes, increasing sophistication among illicit chemists, and the rise of open-source chemical data all combine to create a landscape that’s as complex as ever. For labs, manufacturers, and policymakers, balancing access with responsibility means investing in better education, smarter analytics, and a sharper focus on real-world impact—either in medicine, public health, or law enforcement.
1-Benzoylpiperazine HCl, often called BZP for short, first caught attention as a research chemical. Chemists originally looked into it because it shows interesting activity at neurotransmitter sites in the brain. Some lab research points to similarities with amphetamine-like stimulants. Over time, people experimenting outside regulated labs tried to chase after the stimulating effects labeled as “legal highs.” The once-obscure molecule slowly picked up a reputation in certain circles, but that shift triggered a wave of scrutiny and new laws.
The interest in compounds like BZP through the 1990s and early 2000s mostly came from how they affect the central nervous system. Scientists sought new compounds for possible treatments—fatigue, depression, even studies connected to cognitive enhancement. In my experience reading research from that era, there’s always a sense of cautious optimism. People want to find non-addictive ways to treat mental and neurological problems. But unregulated recreational use, and the unpredictable effects that come with it, quickly sideline any medical promise.
The main non-lab use of BZP has been as a party drug. Folks sold it in pill form, sometimes mixing it with other substances, and promised users a rush of energy and euphoria. The effects turn up because BZP causes the brain to release more dopamine and serotonin, mimicking some of the chemical signatures of stimulants like MDMA or amphetamine. But chasing those effects isn’t a simple story of fun and no consequences. Reports began stacking up—anxiety, high blood pressure, serious hangovers, and even seizures in some cases showed up in emergency rooms. By looking at countries like New Zealand and the UK, we see how quickly regulations moved once doctors started flagging those incidents.
My background in pharmacy taught me to never underestimate how quickly something promising can go sideways if the public grabs hold before researchers have full answers. BZP sits squarely in that category for me. The World Health Organization and many national agencies, including the US DEA, ended up scheduling BZP as an illegal substance. Today, its sale and distribution as anything other than scientific reagent face heavy penalties in most developed countries. Safety always needs to come first, and BZP’s unpredictable side effects put a stop to any recreational future. Still, researchers remain careful to separate scientific study from public misuse.
For anyone looking for stimulation, focus, or relief from mood issues, BZP is a dead end. Validated treatment from a medical professional will always offer better results and greater safety. If the goal is advancing brain science, strict controls with open reporting and peer review matter even more. There’s always pressure to push forward with new molecules, but shortcuts have led to enough heartbreak already. For people tempted to experiment, the low cost out front rarely matches the health cost later. Lawmakers got this one right—the risk is real, and public health needs protecting, even at the expense of curiosity or novelty.
People might look at 1-Benzoylpiperazine HCl on a chemical supplier’s website and wonder if it’s legal. The law can be anything but straightforward, and legality depends on location, intent, and a tangled web of regulations. The name itself doesn’t sound all that threatening. Dig deeper, though, and a different picture appears.
Known among chemists as BZP’s close cousin, 1-Benzoylpiperazine in its hydrochloride form has a record. Once considered for its stimulant effects, recreational users sought it out as a “party pill.” It appeared on the scene around the same time as BZP. This history put it on the radar of lawmakers and resulted in its ban or restriction in several places.
In the United States, the Drug Enforcement Administration assesses chemicals for potential abuse. While BZP numbers among the substances banned nationwide, 1-Benzoylpiperazine itself does not, at least not federally. This creates confusion. It might skirt federal bans, but states and some localities move quicker than Washington. If law enforcement tests a compound and feels the structure matches that of a banned drug, they apply what’s called the Federal Analogue Act. This law treats “substantially similar” chemicals as illegal if sold for human consumption.
I’ve seen folks order chemicals, thinking the absence of a federal ban means a green light. The reality is different. Customs looks at packages, and if the information hints at human consumption, there’s risk. For instance, labeling or intent discussed in emails can influence charges. Simply holding a package with 1-Benzoylpiperazine inside might lead to legal trouble, especially if prosecutors decide to use the analogue law.
European countries tend to take a more sweeping approach. The UK includes 1-Benzoylpiperazine under its Misuse of Drugs Act. Australia and New Zealand both cite risks and keep it off the legal market. Even if a website promises shipping, that’s not a promise of legality on arrival.
Almost anyone can order research chemicals online but living with the cloud of potential prosecution doesn’t make it worthwhile. No one wants a knock at the door over a chemical sent in an unmarked envelope. Law enforcement doesn’t always move in slow motion, either.
Beyond the law, imports from international suppliers might skirt regulations at the source, but they face strict oversight on arrival. Researchers who purchase for legitimate study keep records, assure proper storage, and consult with compliance departments. Hobbyists or everyday buyers rarely have that kind of safety net.
If you’re ever unsure about legal status, an attorney who understands drug law can untangle the details. Speaking from experience, researching a chemical’s background and current legal treatment takes more than a quick web search. Sites claiming “research chemical” legality often operate in a gray or outright black market.
For those interested in science, many reputable companies provide legally approved chemicals. Joining a university lab or another regulated setting keeps risk in check. The world of chemical supply grows fuzzy by design, but clear answers keep people out of harm’s way.
Just because you can hover over a “buy now” button doesn’t mean the law sits on your side. Ask questions, look up current regulations, and if anything looks too good to be true, trust your gut. Staying curious makes sense, but carrying caution never hurt either.
Storing chemical compounds always calls for a bit of know-how. Not every substance gets along with common room conditions. 1-Benzoylpiperazine HCl brings its own set of quirks, and based on my background handling specialty reagents, ignoring these details could mean headaches for the next experimenter. I’ve seen degradation of valuable stock in shared university fridges, and I’ve learned the cost is not just money—it’s time, lost data, and sometimes personal safety if a decomposition product turns out toxic.
Most lab-grade compounds prefer cool and dry quarters, and 1-Benzoylpiperazine HCl fits the bill. High humidity invites clumping and hydrolysis, slowly turning a crystalline white powder into an unreliable mess. Store it in an airtight, properly labeled container, tucked away from wet work. Standard practice among lab techs and industry professionals places this salt at 2–8°C, well within the range of a good refrigerator. No need for the deep freeze, just avoid heat spikes; they trigger breakdown over time. Direct sunlight speeds up the process, and leaving the bottle near a window or heat source becomes a silent mistake.
Compounds like this one, especially when packaged as a hydrochloride salt, act tough but start to fade fast if left exposed. Oxygen in the air and stray UV rays push unwanted side reactions that, after months, can skew purity. I keep sensitive chemicals sealed, away from open air, and never leave the cap off for longer than it takes to remove what I need. Working in academic labs and pharma, I’ve seen careless storage ruin batch consistency—something QA departments track meticulously to avoid unnecessary recalls or failed batches.
Plastic containers too often allow moisture creep. For something as picky as 1-Benzoylpiperazine HCl, a dark amber glass bottle blocks light and resists chemical leaching. Screw caps with a PTFE liner add another useful layer. Labels matter—not only for safety, but to prevent the “who left this here” problem that costs time and sometimes safety. Good labels include the date received, who opened it last, and expiration, kept in plain English.
Work in a clean area, not the general-use bench. Use clean spatulas or dedicated scoops, and keep the bottle opening away from chemical dust. A mistake some make: using the same spatula for different salts, mixing residues, then wondering why their synthesis results drift. Even small impurities change reactivity, especially at the scale most research settings operate.
Proper storage doesn’t just protect the compound; it protects every downstream process. Small-scale pharmaceutical production or research programs face higher costs and more rigorous audits than ever. The smarter, safer choice is always to invest five minutes labeling and storing compounds right. It preserves active life, keeps the material reliable, and saves everyone headaches down the line. As someone who has run both teaching labs and R&D sites, I’ve seen the difference this makes—not just for safety, but for real, measurable productivity.
Once reliable storage habits set in, the whole operation runs smoother. Labs can stretch budgets, researchers trust their reagents, and repeatable results become the norm. It’s a cycle that rewards attention to detail. For 1-Benzoylpiperazine HCl, the lesson is clear: keep it cool, dry, sealed, and labeled. That’s the best path toward safe, predictable outcomes.
1-Benzoylpiperazine HCl, often called BZP, shows up as a chemical that some people experiment with for its stimulating effects. I remember reading how it started getting attention as a sort of “legal high.” People often compared it to amphetamines, but just being “legal” for a while did not make it risk-free. Even after laws caught up in several countries, BZP kept appearing in party scenes. The promise of a quick boost or altered perception can pull in curious users, but many overlook how things can quickly go wrong.
BZP targets the brain’s chemical messengers, especially dopamine and serotonin. I’ve seen reports from users complaining about jitteriness, trouble sleeping, and pounding hearts. Elevated blood pressure and headaches seem common, and a high body temperature sneaks up without much warning. These reactions mean the body is getting worked up far more than it should.
Reality hits when people start feeling agitated or anxious long after the buzz wears off. Jaw clenching and muscle tightness often pop up in emergency room visits tied to this drug. Nausea and vomiting cross into the picture. For those with underlying health issues, the spikes in heart rate can spiral into dangerous territory pretty fast. The body’s stress response keeps firing, putting long-term health at risk.
Stimulants like BZP can push mental health to the edge. I remember reading medical case reports describing panic attacks, hallucinations, and even seizures after using BZP. Regular use brings on paranoia and episodes that leave people questioning what’s real. Mood swings linger even after the physical side effects fade. There’s mounting evidence linking BZP to neurotoxicity—damage to nerves that can be permanent, not just passing discomfort. The way this substance ramps up neurotransmitter release might look harmless at first, but the brain struggles to re-balance, leaving lasting scars.
In real life, folks rarely use BZP on its own. Mixing it with alcohol, MDMA, or other party drugs happens a lot at clubs and raves. That’s where things quickly turn risky. Interactions between substances lead to rapid overheating, severe dehydration, and far higher chances of seizures or heart complications. Once, while working in public health, I saw how these polydrug situations landed even young, fit people in the ICU. There’s no safe formula for stacking drugs, and the body pays the price.
Stronger education drives better choices. Clear facts about the true risks of chemicals like BZP help people decide with their eyes open, not just curiosity. Testing programs at festivals and honest conversations strengthen community responses. Healthcare workers benefit from up-to-date protocols that flag BZP toxicity right away. Most importantly, supportive recovery resources—without judgment—open doors for those who want out. The spotlight must stay on the real risks, helping people sidestep preventable harm.
Every time I see a bottle labeled 1-Benzoylpiperazine HCl, I think about the nature of chemicals that combine unpredictability with subtle hazards. This compound doesn’t often make headlines, yet it serves as a reminder that so-called “routine” reagents can pose risks if handled carelessly. Navigating the laboratory with such substances always demands a blend of vigilance and practical sense.
Nobody wants to end a day at the bench with irritated skin or worse. I always suit up before working with 1-Benzoylpiperazine HCl: lab coat fully buttoned, goggles snug, gloves on both hands. Nitrile gloves deliver reliable protection since this compound can pass through some thinner latex. The importance of closed-toe shoes hit home years ago after watching a spill splatter across a classmate’s sneakers—they learned the hard way that feet are as vulnerable as hands.
Some believe that a chemical lacking a strong odor or volatility doesn’t require a fume hood. It’s easy to fall into that trap. While 1-Benzoylpiperazine HCl isn’t wildly volatile, procedures such as weighing or transferring the powder can kick particles into the air. I use a certified fume hood for these tasks, never trusting open air. Research from NIOSH backs up this habit, showing that enclosed air systems reduce accidental inhalation of substances, even for low-vapor chemicals.
Lab training hammered home the old lesson: never touch, taste, or directly smell any compound. 1-Benzoylpiperazine HCl absorbs through the skin, and a small spill can turn a clean workspace into a headache for everyone. I dedicate separate spatulas and weigh paper for handling the powder, never returning excess back to the original container. Once, a labmate’s cross-contaminated scoop set off a cascade of cleanup procedures; one careless moment can trigger costly downtime.
Cleanup habits shape laboratory safety. I wipe down work surfaces as part of my closing routine, using the right solvents or just soapy water for non-reactive residues. Waste containers labeled for organic solids handle contaminated gloves, weigh paper, and small debris. Larger spills call for a specific kit—vermiculite and absorbent pads, sometimes a neutralizer depending on the protocol. Regulatory guidance from the EPA and local environmental offices offers practical instructions for hazardous waste pickup, which overrides guesswork that could endanger janitors or sewer systems.
No manual can replace hands-on training. I learned early by shadowing a chemist with decades of experience. Their steady approach to each bottle—never rushing, always checking labels twice, sharing stories about mishaps—proved as valuable as anything in print. Group safety meetings keep everyone aware, especially with updates about chemical toxicity or changes in disposal rules. Sharing those lessons consistently builds a culture where new and old staff look out for each other.
1-Benzoylpiperazine HCl isn’t the most dangerous chemical in any storeroom. Yet real safety depends on a habit of vigilance and a respect for risk. Taking shortcuts usually catches up, sooner or later. With simple precautions—personal protection, ventilation, careful handling, good cleanup, and constant learning—I find that even among hundreds of bottles in a storeroom, I never lose sight of what matters most: getting through the day safely and returning home with nothing but clean hands and a clear mind.
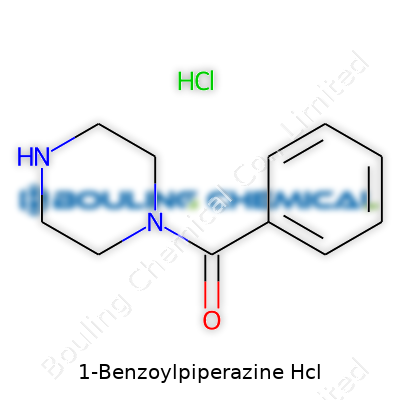
| Names | |
| Preferred IUPAC name | 1-(2,3-Dihydro-1H-phenyl-1,4-diazepin-1-yl)ethan-1-one hydrochloride |
| Other names |
1-Benzoylpiperazine hydrochloride 1-Benzoyl piperazine HCl NSC 275219 N-Benzoylpiperazine hydrochloride |
| Pronunciation | /wʌn bɛnˈzɔɪl paɪpəˌreɪziːn ˌeɪtʃ-siː-ɛl/ |
| Identifiers | |
| CAS Number | 5322-33-6 |
| 3D model (JSmol) | `3D_structure;JSmol;C1CN(CCN1)C(=O)C2=CC=CC=C2.Cl` |
| Beilstein Reference | 1717785 |
| ChEBI | CHEBI:131346 |
| ChEMBL | CHEMBL2105935 |
| ChemSpider | 21733617 |
| DrugBank | DB11363 |
| ECHA InfoCard | ECHA InfoCard: 100947-458-2 |
| EC Number | EC 212-082-2 |
| Gmelin Reference | 386846 |
| KEGG | C14379 |
| MeSH | D02.241.223.211.125 |
| PubChem CID | 102406828 |
| RTECS number | VS2900000 |
| UNII | 61CF4T8GKK |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | CXT14D3U8J |
| Properties | |
| Chemical formula | C11H15ClN2O |
| Molar mass | 266.76 g/mol |
| Appearance | white solid |
| Odor | Odorless |
| Density | 1.14 g/cm3 |
| Solubility in water | Soluble in water |
| log P | 1.08 |
| Acidity (pKa) | 9.8 |
| Basicity (pKb) | 2.8 |
| Dipole moment | 3.85 D |
| Pharmacology | |
| ATC code | N05CM22 |
| Hazards | |
| Main hazards | May cause respiratory and skin irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | CN1CCN(CC1)C(=O)C2=CC=CC=C2.Cl |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P273, P280, P301+P312, P302+P352, P305+P351+P338, P312, P330, P337+P313, P362+P364 |
| LD50 (median dose) | LD50 (median dose) for 1-Benzoylpiperazine HCl: 210 mg/kg (rat, oral) |
| NIOSH | Not Listed |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10g |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
N-Benzylpiperazine 1-(2-Methoxybenzoyl)piperazine 1-(4-Fluorobenzoyl)piperazine 1-(3,4-Methylenedioxybenzoyl)piperazine 1-Phenylpiperazine |