1-Benzhydrylpiperazine came onto the scene in the early 20th century as chemists dove into piperazine derivatives. The growth of pharmaceutical research in post-war Europe saw researchers searching for new psychoactive substances and intermediates, which pushed benzhydrylpiperazine into a spotlight. Many early reports focused on how the piperazine backbone could combine with aromatic groups such as benzhydryl, opening up a new world for organic synthesis and medicinal chemistry. The compound found use as a building block for a range of new drugs and research chemicals, its flexibility making it a staple for those tinkering in medicinal chemistry labs. Over time, big pharma and university teams both started probing its pharmacological properties, looking for new antihistamines, antidepressants, and even non-addictive anxiolytic options. Its chequered journey includes flags in regulatory circles, reflecting a tug-of-war between innovation and safety.
1-Benzhydrylpiperazine offers a molecular structure tough enough to survive storage and shipment but adaptable enough to anchor chemical synthesis projects. The piperazine ring with its two nitrogen atoms works as a sturdy core, and once you tack on the bulky benzhydryl group, you get a compound ready for both industrial and boutique-scale labs. It’s available in powder or crystalline forms, usually trading hands in grams or kilograms depending on whether it's for academic research or commercial supply. Its chemical flexibility has encouraged both professional chemists and students to reach for it in developing new drug candidates, intermediates, and specialty materials.
Crystalline and odorless, 1-Benzhydrylpiperazine typically appears as a white solid. The compound melts just below 170°C and dissolves readily in organic solvents like ethanol and dichloromethane. Water solubility runs low, which can complicate some biological applications but simplifies extractions and purifications after reactions. The molecule stands stable at room temperature if kept away from acids and strong oxidizers. At a molecular weight of about 266.37 g/mol, the compound brings enough heft for handling and separation while keeping solutions manageable in high-throughput synthesis setups. Its structure, with a central piperazine ring sandwiched by a benzhydryl moiety, gives it both basic and lipophilic properties—a combination that chemists leverage when preparing derivatives or exploring receptor interactions in pharmaceutical research.
Suppliers routinely label shipments with chemical identity, batch number, and purity—often at 98% or above—as well as hazard pictograms reflecting the basic risk profile. Certificates of analysis arrive with every batch, pinpointing melting points, impurity profiles (usually by HPLC or GC), and spectral data, such as NMR or IR. Many users request an MSDS alongside shipment papers, laying out proper storage, personal protective equipment, and first aid recommendations for accidental contact. Regulatory numbers like CAS 92-54-6 and UN identifiers help with customs and record-keeping, keeping operations transparent and compliant with safety ordinances. Companies pay close attention to terrestrial and local transport rules, given that piperazine derivatives sometimes fall under controlled categories or require extra paperwork for customs clearance.
Labs typically generate 1-Benzhydrylpiperazine using classic alkylation. A two-stage process kicks off with benzhydryl chloride (or bromide) mixing with piperazine under basic conditions, often in solvents like acetonitrile or toluene. These reactions might use potassium carbonate or similar bases to mop up the liberated acid, keeping the solution flowing smoothly. Careful cooling limits unwanted byproducts, and the reaction runs with vigorous stirring to get good contact between phases. Most post-reaction mixtures undergo extraction, sometimes by liquid-liquid separation or even column chromatography. To remove polar byproducts, washing with brine or dilute acids helps, followed by drying and evaporative concentration to recover the target solid. Recrystallization with solvent mixtures like ethanol-water sharpens up the yield, delivering the high-purity product needed for pharmaceutical or analytical testing.
The piperazine ring at the heart of this compound puts up with a range of chemical modifications. Reductive amination, acylation, and N-alkylation remain common moves, allowing chemists to customize biological activity or produce tagged derivatives for analytical work. The benzhydryl group tolerates mild oxidizers, and it’s not unusual to see halogenation or functionalization on the aromatic rings. Labs create salt forms—involving succinates, hydrochlorides, or acetates—for tailored solubility or stability, especially when downstream formulation demands certain physicochemical traits. Medicinal chemists sometimes modify the benzhydryl substituent, swapping in different meta- or para-substituents to probe receptor binding or metabolic stability, especially in drug discovery setups. The sheer number of theoretical derivatives has pushed many dissertation projects and filled shelves with trial vials over the decades.
Over the years, the chemical has worn a few names besides 1-Benzhydrylpiperazine. Its IUPAC name is straightforward: 1-(diphenylmethyl)piperazine. Some suppliers and research papers refer to it as benzhydrylpiperazine or DPM. Regulators and customs paperwork usually list the CAS registry number 92-54-6, providing clarity among look-alikes. In scientific catalogs, it sometimes sneaks in as Diphenylmethylpiperazine, a name that crops up in patent filings and older pharmacological texts. Street-level chemists and those working in less-official circles sometimes use “Benzydryl-pip” or “DPMZ” shorthand, but major distributors rely on the IUPAC or CAS-linked nomenclature to avoid confusion. This stable collection of names anchors the compound’s reputation in both literature and commerce.
Handling 1-Benzhydrylpiperazine means giving chemical hygiene respect. Avoiding powder inhalation and skin exposure reduces risk, with gloves, goggles, and laboratory coats forming the first line of defense. Spills demand immediate cleaning—preferably with absorbent pads and not just standard tissue, since the compound’s oily friends can spread further than expected. Some jurisdictions classify it under “watch-lists” for psychoactives, so recordkeeping and access control play a part in lab protocols. Reactivity to strong acids or oxidizers also warrants storing away from bleach or nitric acid solutions. Fume hoods see regular use when dissolving the compound in volatile solvents. Trash from synthesis and cleanup runs through licensed disposal, as per hazardous waste regulations, with environmental teams occasionally checking for leaching products in high-usage facilities. The focus stays on keeping accidental exposure low and process transparency high, in line with recent pushes from regulators and occupational safety groups.
In pharmaceuticals, 1-Benzhydrylpiperazine has popped up both as a research compound and as an intermediate for building more complex molecules. Medicinal chemists look to it for leads in antihistamine, antipsychotic, or anxiolytic development. The compound’s structural backbone sets the stage for dozens of clinical candidates, and university teams often use it to generate new ligands for serotonin or dopamine receptors. Veterinary researchers test piperazine derivatives for novel antiparasitic agents, and chemical engineers have tried out benzhydrylpiperazine-based surfactants and materials modifiers in specialized plastics or coatings. Analytical chemists use it as a reference or derivatizing agent, exploiting its sturdy structure. Its strong lipophilicity, combined with water-insolubility, even makes it useful in piloting membrane studies or some experimental delivery systems, though progress there has been patchy.
Making sense of this chemical keeps research teams busy worldwide. Studies investigate how substituents on the aromatic rings affect binding at a range of CNS receptors. Some teams run computational simulations, exploring how tweaks to the molecule shift its interaction profile across enzyme or receptor families. In drug candidate testing, 1-Benzhydrylpiperazine derivatives hit behavioral screens for antianxiety or antidepressant traits, and these results flow into preclinical safety studies. A handful of groups look at it as a backbone for designing PET imaging tracers or custom radioligands, since its chemical toughness makes for high-yielding labeling strategies. Beyond drugs, universities and corporate labs test polymerization and surface chemistry roles, building materials with edge-case applications in electronics and sensor design. By any metric, the research stack keeps mounting, with patent applications and open-access data banks documenting fresh twists on its chemistry.
Toxicologists rank benzhydrylpiperazine as a chemical demanding caution—acute exposure can lead to central nervous system effects. Animal studies show moderate LD50 values, and some analogs have slipped into the designer drug scene, prompting forensic awareness. Dosage and route matter, especially since derivatives sometimes produce pronounced psychoactive effects at higher intake. Rats and mice exposed to significant doses show hyperactivity, respiratory changes, and altered startle response, suggesting dopaminergic or serotonergic involvement. Long-term studies probe impacts on liver and kidney function, with mixed findings depending on metabolism rate and knockout models. Cell culture assays flag cytotoxicity at millimolar concentrations, serving as a red flag for workers handling bulk volumes or running high-concentration reactions. Regulators and ethics panels urge robust risk assessment for new applications, with clear labeling and informed consent at every trial stage, no matter the research setting.
Society stands divided about how far to run with 1-Benzhydrylpiperazine and its relatives. On one hand, the basic skeleton has more to give for CNS drug development, especially as the need for non-addictive anxiolytics and mood stabilizers keeps growing. Advances in computational chemistry let teams narrow down these candidates faster, and the pipeline of analogs remains long. There’s promise in developing imaging agents or specialty polymers, with academic and industrial labs both investing in pilot projects. On the other hand, regulation and rising safety expectations force tighter controls, especially as recreational misuse and environmental footprints draw fresh scrutiny. If science, industry, and regulators collaborate, new uses and safer handling protocols could emerge. The raw versatility of the molecule appeals to those solving both basic and applied chemistry puzzles. This intersection of risk and reward will keep the chemical in headlines and journals for years.
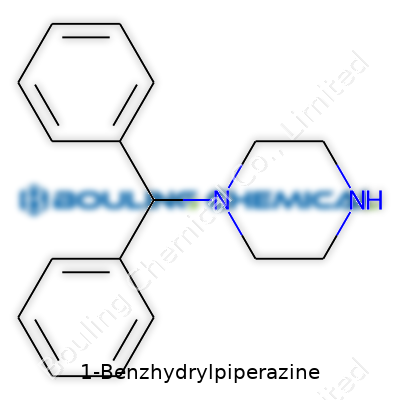
1-Benzhydrylpiperazine might sound like just another tongue-twisting name from a chemistry textbook, but it holds a practical place in both medicine and the world of research. Anyone who’s stumbled through a pharmacy shelf or followed chemical supply catalogues likely ran into names like “diphenylmethylpiperazine.” That’s just another label for 1-Benzhydrylpiperazine. Its structure gives it a piperazine ring attached to a benzhydryl group — a foundation that many synthetic drugs have borrowed over the decades.
Diving into real-life usage, this compound has a key role as a building block for common antihistamines. For example, hydroxyzine and cetirizine, found in allergy pills, both spring from the 1-Benzhydrylpiperazine skeleton. Think about those times when hay fever hits, and one needs an antihistamine to keep symptoms under control. The core structure from this chemical lands in tablets that help people breathe easy and sleep a little better on rough days.
Beyond allergies, some of its derivatives have turned up in anti-anxiety medications. Decades ago, researchers connected this backbone with calming effects in the nervous system. Folks dealing with nerves, sleeplessness, or itchiness sometimes took medicines with a close chemical relationship to this compound. The medical community’s interest hasn’t faded because scientists continue to experiment with tweaks to its structure, hoping for safer, more targeted drugs with fewer side effects.
In research settings, chemists love using 1-Benzhydrylpiperazine as a starting point or “scaffold” for making new molecules. It’s not just about pharmaceuticals. Many researchers reach for it when building ligands for bio-receptors, hoping to unlock new approaches to understanding how our bodies process signals like pain, pleasure, or allergic reactions. These small steps are what lead to the next wave of better medication or treatments for chronic problems.
Every chemical with psychoactive effects draws attention from both the medical world and those looking for a high outside the law. Some derivatives have found their way into recreational use, usually sold as “legal highs” or under-the-counter products. The safety profile for these designer drugs often remains unclear, with reports of side effects like confusion, rapid heart rate, and sometimes dangerous reactions.
Authorities have found themselves in a catch-up game, chasing after newly tweaked versions that skirt regulations. Real lives get caught in the mix. Emergency room visits related to these compounds climbed in some countries, mostly due to lack of information on dosage, effects, and interactions. This shadow side creates a demand for stronger regulations, clear labeling, and more public education on risks tied to these kinds of chemicals.
The story of 1-Benzhydrylpiperazine isn’t just about lab coats and pill bottles. Its journey from pharmacology lab to street corner shows the importance of keeping science moving alongside common-sense policy. Supporting open research, public health messaging, and responsible prescription use might help reduce harm, foster innovation, and make sure compounds like this one stick to roles that truly help people live healthier, safer lives.
Some chemical names just stick with you after a bit of time spent around forums or a university lab, and 1-Benzhydrylpiperazine (commonly known as BZP) falls into that group. For years, BZP popped up in everything from party pills to random online shops. The questions about its legal status have created more web searches and heated debates than you’d think for a compound that looks so simple on the page.
No one-size-fits-all answer exists for the legality of buying BZP. In the United States, federal law made BZP a Schedule I substance back in 2004, meaning it gets treated much like MDMA or LSD: unlawful to possess, sell, or ship, regardless of intended use. This decision came after a rise in recreational use and toxicity cases, especially among young adults who viewed it as a "legal high." Prior to that ban, stores in New Zealand sold BZP as a legal alternative to amphetamines, which drew a lot of college students and curious club-goers. New Zealand later banned it, too, citing health risks and rising hospital admissions linked to its use.
Different countries sit all along the spectrum. Some–such as Australia, Canada, and most EU members–prohibit production and sale. In Europe, multiple countries treat BZP much like controlled pharmaceuticals or street drugs, making even possession a criminal issue. In a handful of places without explicit bans, authorities apply analog laws. That means anything with similar structure or effects to a known controlled substance lands in legal trouble. So, ordering BZP from overseas or importing even tiny amounts means risking customs intercepts, fines, or worse.
Most folks interested in BZP look for a stimulant. According to harm reduction nonprofits and chemistry professors I’ve met, users seek increased alertness or mood elevation, especially when traditional stimulants stay out of reach or get too expensive. The cravings for “legal highs” show up again and again any time a new ban goes into effect. Legal loopholes only shift the marketplace: when regulators block BZP, a new, untested chemical fills the same role, often with more unknown risks.
The quick turnaround between one substance ban and the next legal alternative leads to a cycle. Chemistry labs in countries with weaker oversight can sell molecules to anyone with a credit card. Small shops, online marketplaces, and shady websites promise overnight shipping. Buyers try to read through pages of legal code, crossing their fingers that the substance lands at their door instead of a police evidence locker. I have watched more than one forum member trade caution for thrill, and sometimes that gamble ends with a hospital visit or a court date.
Outlawing specific chemicals won’t slow demand—or curiosity. Fact-based drug education, accessible harm reduction info, and candid mental health support work better than blanket bans. Researchers need clear paths to study the real effects of these substances without fear of prosecution, so doctors can respond to overdose cases with good science and not just anecdote.
Anyone considering buying something like BZP deserves up-to-date legal facts, not rumor. Local drug policy groups, poison control centers, and medical professionals offer better advice than old forum posts or shadowy retailers. With governments constantly updating their controlled substances lists, trying to skirt the law by buying online rarely ends well.
As long as chemicals like BZP exist, so will misinformation and legal headaches. The safest option often means checking real sources—like government websites, pharmacists, or toxicologists—before clicking “add to cart” on chemicals with a checkered past.
Plenty of people look up 1-Benzhydrylpiperazine (also called BZP) trying to figure out if it’s safe to use. BZP once showed up in so-called party pills across New Zealand, parts of Europe, and North America. Headlines about seizures and even hospitalizations get people worried about what risks actually come with this drug. Doctors and toxicologists steer the conversation away from hype and push folks to look closely at what the research says, because real lives hang in the balance.
The experience most people talk about starts with a burst of energy, a bit like the rush of having too much caffeine. Users often mention headaches, nausea, and sometimes trouble catching their breath. Sleep does not come easily after taking BZP; tossing and turning at three in the morning stops being funny pretty fast. Appetite goes out the window as energy jumps and heart rates speed up—some people even feel a tingle or a chill crawling up the back of their neck.
It doesn’t end with feeling jittery. Emergency rooms have treated people for much more serious problems after using BZP. Seizures have sent healthy teenagers and adults straight into urgent care, sometimes before they figure out what's happening. Sweating buckets, chest pain, fast or pounding heartbeat, and dangerously high blood pressure fall into a category that no one wants to roll the dice with. Some folks end up confused or, in rare cases, hallucinate. A research survey in the UK showed BZP mixed with alcohol pushed some users into a confused, hyperactive state leading to panic or aggression—adding fuel to the fire for both body and brain.
BZP targets chemicals in the brain linked to mood and reward. That chemical meddling brings mood swings—excitement turns to anxiety or agitation, and bad days feel even worse. Stories from hospital records and poison control centers connect BZP use with paranoia and even psychotic symptoms. For someone sitting on the edge of anxiety or depression, this drug will not help. It can make mental health symptoms sharper and tougher to manage. The World Health Organization flagged reports of both short-term and lingering mood changes, even after only a couple of uses, giving health professionals more reasons to sound the alarm.
No amount of BZP can be called safe. People with heart issues, high blood pressure, or mental health conditions end up with a bigger target on their backs. Young people, who might try these pills thinking they only promise a night of fun, sometimes don’t see the danger until it’s sitting right in front of them. The lure of something new or different sparks curiosity, but experience tells us that the price for a few hours of excitement can show up as weeks or months of health problems.
Clear information and honest talk matter more than ever. Reaching people through credible sources—family doctors, school counselors, or trustworthy health websites—gives them room to ask the hard questions. Some countries have chosen to ban BZP completely. For those worried about what’s in a drug they are offered, drug-testing kits might help, though they never guarantee protection from possible harm. Open conversations and consistent access to mental and physical health support change the stories that make headlines.
Dealing with chemicals like 1-Benzhydrylpiperazine brings responsibility. This compound, often used in research and found in some chemical synthesis projects, shows how standards in storage affect safety, health, and results. My years working in laboratories taught me that even everyday routines with chemicals can expose people to risks if ignored or done carelessly.
Anyone who’s spent long hours in a lab knows how much can go wrong with improper storage. It’s too easy to reach for something and discover you’ve got degraded material or a corroded cap. For 1-Benzhydrylpiperazine, a cool, dry spot makes sense. Moisture and heat invite trouble — both can change a chemical’s stability, destroying its value or even raising the odds of a reaction that escapes attention until it’s too late. Direct sunlight should stay off the list. More than once, I’ve seen clear bottles on windowsills change color or form odd crystals, all because someone skipped this simple rule.
Glass containers with tight seals set the standard for storage. Plastic can work, but only with confirmation it won’t react with the contents. Anything with a loose lid or an unknown material invites problems. Chemicals have ruined storage shelves because their vapors escaped from old, cracked containers. Using proper labels – with both the chemical name and the date opened – seems obvious, but I can’t count how often I’ve been left guessing because someone used shorthand or dropped the pen halfway.
Rules for segregation exist for reasons rooted in hard experience. Mixing bases, acids, and oxidizers in one place adds a layer of risk few want to face. I always keep piperazines away from strong acids and oxidizing agents. Chemical accidents rarely happen in neat, controlled explosions; they sneak up as leaks, unseen vapors, or cross-contamination. Even if nothing dramatic occurs, improper segregation affects purity. This could waste a week’s work in a research setting.
Expired or degraded chemicals sneak up as silent threats. At one job, we kept a log to monitor stock — not out of bureaucracy, but because expired 1-Benzhydrylpiperazine had attacked a cap, leaking an irritating smell. With a regular schedule, expired compounds get pulled before trouble starts. Safe disposal matters just as much as storage. Local regulations guide this part; never dump leftovers down the sink or in the regular trash. Responsible labs call in qualified disposal services. People sometimes ignore cost, not thinking about long-term consequences for themselves or their colleagues.
Teaching newcomers to the lab starts with drumming in respect for these routines. Tired habits and shortcuts don’t mix with potentially hazardous materials. I usually spread stories about what went wrong and why, hoping experience takes root rather than just rules. If companies and organizations build a culture of safety and knowledge-sharing, everyone benefits — from the intern to the veteran technician. Simple measures — solid containers, segregated shelves, logs, reminders — can make the difference between smooth work and dangerous situations. Long-term, these habits stack up: fewer accidents, less waste, better outcomes for everyone.
Few people walk into a pharmacy searching for 1-Benzhydrylpiperazine. This compound, not commonly seen in mainstream medication, tends to slip under the public radar. Pharmacists don’t stock it on regular shelves, and searching for it in Western medicine drawers leads nowhere. Instead, it’s frequently discussed in another context—chemistry circles, scientific literature, and, sometimes, law enforcement briefings.
1-Benzhydrylpiperazine itself began as a chemical curiosity with no well-established medical purpose approved by global regulatory bodies like the FDA or EMA. Researchers might use it in controlled lab settings. No reputable doctor writes a prescription for it because it doesn’t hold a recognized therapeutic use. So walking into a clinic and requesting it won’t yield a prescription pad coming out of the doctor’s pocket.
Legal systems often move faster with certain substances than doctors do. 1-Benzhydrylpiperazine has earned attention not due to benefit but due to risk. Decades back, its cousins—compounds in the piperazine family—started cropping up in recreational use. Some countries noticed public health concerns as individuals reported unwanted side effects and toxic reactions after using these substances at parties or mixed with other drugs. Right now, many governments restrict or outright ban its use, sometimes as part of broader bans on “designer drugs.”
In the United States, the Drug Enforcement Administration included some piperazine compounds on the controlled substances list. The UK placed many related compounds under the Misuse of Drugs Act. Australia treated the whole class of chemicals with caution, slapping bans at the federal and state levels. Each move came after patterns of abuse and health emergencies started growing. These laws hit hard—meaning possession or intent to sell can land someone in trouble, regardless of intent.
Rules swing widely depending on which border you cross. Most Western countries do not even allow pharmacies or doctors to hand out 1-Benzhydrylpiperazine because no approved medical use exists. If you look through the official drug handbooks, the name goes missing. Instead, authorities often list it with prohibited substances. So, in places like the US, Canada, or most of Europe, nobody writes a prescription or dispenses it behind the counter.
That said, nothing stops some nations from tightening or relaxing restrictions over time. I’ve seen how chemical regulations change quickly. Ten years ago, many provinces in Asia left so-called research chemicals untouched. Now, after health scares and hospitalizations, rules are changing.
Despite chemistry’s appeal, not every molecule lands in the medicine drawer. 1-Benzhydrylpiperazine fell into the spotlight for the wrong reasons. Health agencies have flagged it for potential harm—ranging from seizures and heart irregularities to dangerous behavior when mixed with alcohol or stimulants.
Public education can help people understand why some chemicals don’t belong in home pharmacies. Clear information from health departments gives parents and young adults the tools to avoid risks. Legislators should continue working with scientists to assess new compounds as they emerge, tightening controls swiftly if early signs of harm appear. Doctors, pharmacists, and anyone working in public health play a key role in identifying trends early and getting facts out before misinformation takes hold.
Doctors and pharmacists rarely see requests for 1-Benzhydrylpiperazine, but questions about new drugs or supplements keep coming. Each answer relies on updated evidence, transparency, and honest risk assessment. Open conversations can help those curious about lesser-known compounds find safer, well-tested alternatives. Telling the stories behind bans—whether they involve chemistry labs, hospitals, or courts—delivers more than rules. It helps people understand real dangers and choose safety first.
| Names | |
| Preferred IUPAC name | 1-(Diphenylmethyl)piperazine |
| Other names |
N-Benzhydrylpiperazine Diphenylmethylpiperazine Piperazine, 1-diphenylmethyl- 1-(Diphenylmethyl)piperazine |
| Pronunciation | /wʌn ˌbɛnzˈhɪdrɪl paɪˈpɛrəziːn/ |
| Identifiers | |
| CAS Number | 92-24-0 |
| 3D model (JSmol) | `3D_model_JSmol="C1CN(CCN1)C(c2ccccc2)c3ccccc3"` |
| Beilstein Reference | 1209227 |
| ChEBI | CHEBI:132537 |
| ChEMBL | CHEMBL418 |
| ChemSpider | 16282 |
| DrugBank | DB04161 |
| ECHA InfoCard | 13d181b8-7e0e-42ba-9e4d-7ff7b2c2d0b2 |
| EC Number | 205-592-2 |
| Gmelin Reference | 170724 |
| KEGG | C14314 |
| MeSH | D010574 |
| PubChem CID | 71704 |
| RTECS number | TP4550000 |
| UNII | 6LR8C1B02N |
| UN number | UN3077 |
| Properties | |
| Chemical formula | C17H20N2 |
| Molar mass | 324.45 g/mol |
| Appearance | White to off-white crystalline powder |
| Odor | Odorless |
| Density | 1.1 g/cm³ |
| Solubility in water | slightly soluble |
| log P | 3.6 |
| Vapor pressure | 1.46E-4 mmHg at 25°C |
| Acidity (pKa) | pKa = 9.73 |
| Basicity (pKb) | 3.67 |
| Magnetic susceptibility (χ) | -88.74·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.619 |
| Viscosity | Viscous oil |
| Dipole moment | 3.05 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 418.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | Unknown |
| Pharmacology | |
| ATC code | N04BX08 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes serious eye irritation, may cause respiratory irritation |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | Precautionary statements of 1-Benzhydrylpiperazine: "P261, P264, P271, P272, P280, P302+P352, P305+P351+P338, P308+P313, P337+P313, P362+P364, P405, P501 |
| Flash point | 77.6°C |
| Lethal dose or concentration | LD50 (oral, rat): 210 mg/kg |
| LD50 (median dose) | LD50: 210 mg/kg (rat, oral) |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 1-Benzhydrylpiperazine: Not established |
| REL (Recommended) | 100-500 mg |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Benzylpiperazine Diphenylmethylpiperazine 1-Phenylpiperazine 1-(2-Benzhydryloxyethyl)piperazine |