Many decades back, chemists searching for new building blocks stumbled on the pyrrolidine ring, a five-membered structure with a nitrogen atom. That alone got plenty of attention for its usefulness in drugs and agrochemicals, but things changed once researchers figured out how to attach an amine group directly. 1-Aminopyrrolidine, sometimes called aminopyrrolidine or 1-APD, drew interest from both pharmaceutical chemists and industrial synthesis teams. Efforts to optimize production techniques surged through the eighties, often mirroring the history of other amine-based compounds: lab-scale curiosity, then scale-up driven by need, often for specialty processes or promising new drugs.
1-Aminopyrrolidine serves as an intermediate in pharmaceutical and fine chemical syntheses. Its compact structure and reactive amine group provide an edge over bulkier or less accessible amines. Demand for this molecule comes from labs focused on heterocyclic or chiral compound synthesis. It’s the backbone of various therapeutic candidates tested in the last twenty years, and manufacturers value the way it unlocks unique transformation steps in organic chemistry.
The compound appears as a clear to slightly yellow liquid at room temperature, with a faint ammonia-like smell familiar to anyone who’s handled small amines. It boils in the range of 150–160°C, which enables separation by fractional distillation if you happen to need a pure cut. It dissolves readily in water, ethanol, and common polar solvents, thanks to the small ring and exposed amine group. Chemically, 1-Aminopyrrolidine shows strong basicity, and its ring structure resists isomerization and decomposition under lab conditions. Reactivity springs from its free amine, making it a valuable nucleophile in coupling or reductive amination reactions.
Standard bottles or drums carry hazard labels warning about corrosiveness and inhalation risk, with GHS pictograms you’d expect for amines, and the CAS number 930-49-2 for regulatory tracking. I’ve seen technical-grade product offered as 98% minimum purity with water content below 1%, but research labs often look for 99% purity, since trace moisture promotes hydrolysis in sensitive syntheses. Manufacturers indicate batch number, lot history, and test results for amine value or residual solvents right on the certificate of analysis. This level of traceability lets process chemists flag problems before they hit scale-up.
Historically, preparation used the reductive amination of pyrrolidone with ammonia or a primary amine under hydrogen and a suitable catalyst like Raney nickel or palladium on carbon. Additional approaches swap out precursor compounds for better yields or fewer side products. The continuous flow processes some companies use cut out batch-to-batch variability, which is helpful for pharma firms that rely on the intermediate in regulated workflows. Purification usually involves fractional distillation under vacuum, sometimes followed by drying over potassium carbonate if dryness is critical.
The compound’s most useful reaction feature is its primary amine. It undergoes condensation, alkylation, and acylation—useful for making Schiff bases, amides, or secondary amines. Chemists also use it in ring-opening reactions with anhydrides and for cyclizations leading to new heterocyclic scaffolds. In pharmaceutical development, it gets tested for stereoselective modifications to fit the process at hand. Its pyrrolidine ring resists harsh conditions, so it survives steps that break down less robust amines. I’ve seen colleagues develop ligands or catalysts based on 1-aminopyrrolidine skeletons, exploiting both its nucleophilicity and rigidity.
You’ll find 1-Aminopyrrolidine sold under various names depending on the supplier. The IUPAC name is straightforward, but documentation lists alternatives like Pyrrolidin-1-amine, 1-pyrrolidinamine, 1-Amino-pyrrolidine, and N-Aminopyrrolidine. Some catalogues list it alongside structurally related materials, so careful attention to the full CAS number—930-49-2—matters for ordering and compliance, especially in pharmaceutical workflows.
Safe handling means more than gloves and goggles. The compound’s volatility at room temperature can catch you by surprise, and it gives a sharp odor that can irritate mucous membranes even in trace airborne quantities. Direct contact leads to chemical burns if left on the skin and, in my experience, uncontrolled spills linger in the lab unless handled quickly. Standard operating procedures lay out steps for decontamination, spill handling, and waste disposal consistent with local rules. Storage in cool, dry, well-ventilated spaces in tightly sealed containers prevents decomposition and accidental release. MSDS documentation notes incompatibility with strong oxidizers and acids.
Pharmaceutical development accounts for a big chunk of global demand. Chemists employ 1-aminopyrrolidine as a raw material for active pharmaceutical ingredients (APIs), often as a precursor to highly functionalized pyrrolidine derivatives seen in everything from antivirals to central nervous system drugs. Agrochemicals companies use it to construct complex synthetic intermediates, including advanced insecticides and herbicides. Materials scientists sometimes explore its reactivity as a monomer in specialized polymer synthesis, where amine-functionalized chains confer thermal and mechanical resilience. Custom synthesis firms keep it on hand for research clients testing proprietary heterocyclic molecules and specialty ligands.
Current research keeps turning up new roles for 1-aminopyrrolidine. Medicinal chemists screen analogues for biological activity against oncological and neurological targets, benefiting from the molecule’s flexible modification options. Teams exploring 'green chemistry' routes target milder, more selective synthetic approaches, dropping out metal catalysts and hazardous reagents in favor of enzymatic or photochemical conversions. Lab notebooks fill up with optimization data on catalyst choice, yield, and selectivity. Drug discovery firms value 1-aminopyrrolidine as a way to rapidly append basic nitrogen atoms within potential drug scaffolds—an advantage in hit-to-lead campaigns looking for safe, bioavailable compounds.
Limited animal studies suggest moderate acute toxicity by ingestion or skin absorption. In my years around synthetic labs, I’ve seen how exposure risks rise with volatility and the basicity of the amine. Inhalation can cause respiratory discomfort and high exposures aggravate central nervous irritability. Chronic toxicity data is sparse, so researchers and process engineers stick to strict exposure controls, thorough ventilation, and prompt spill clean-up. No links to mutagenicity or reproductive toxicity have reached public databases, though absence of evidence should not lead to complacency in high-volume settings or drug formulation work.
As pharmaceutical development leans on richer libraries of small molecular fragments, demand for versatile amines like 1-aminopyrrolidine only grows. Synthetic efforts keep driving yields higher and waste lower, both for cost and to meet tightening environmental requirements. A shift toward biocatalytic production methods might lower overall hazard ratings and lower environmental impacts. Regulatory scrutiny will push for ever-better data on exposure effects and downstream waste products. Companies that deliver on green synthesis, robust documentation, and reliable supply often win out, based on watching years of trends repeat across the fine chemical industry. For chemists working at the intersection of basic building blocks and cutting-edge discovery, molecules like 1-aminopyrrolidine offer both practical and strategic value.
1-Aminopyrrolidine stands out in the world of chemistry for more than just its hard-to-pronounce name. Its chemical formula, C4H10N2, tells a story about structure and potential applications. Breaking this formula down, you’ve got four carbon atoms, ten hydrogen atoms, and two nitrogen atoms locked together in a small yet fascinating molecular ring, with an amino group attached. For a lot of us who’ve ever spent their afternoons in the lab, just seeing that formula brings back the smell of solvents and the click of glassware.
Picture yourself trying to develop new pharmaceuticals or searching for chemical intermediates that won’t break the budget. Sometimes, all you have to work with is a printed formula like C4H10N2. Every number and letter points toward how molecules fit together and how they can react. In the case of 1-Aminopyrrolidine, that fairly straightforward formula means someone can easily draw the structure, map out expected reactivity, or predict if it’ll dissolve in water.
1-Aminopyrrolidine forms part of a small family known as substituted pyrrolidines, which often show up in research around new medicines. Its basic formula means it tends to act as a building block in creating bigger, more complicated molecules. In drug design, ring structures with nitrogen atoms often help tweak how a compound behaves in the body, sometimes improving absorption or stability. Chemists learn to look at a formula and catch these little cues, which helps them figure out what new compounds might work as future treatments.
Anyone who’s ever worked in a lab knows that even something as small as an extra hydrogen or nitrogen atom can move a chemical from “safe to handle” to “definitely glove and goggles territory.” Looking at C4H10N2, you spot the hints that 1-Aminopyrrolidine can be a bit of a troublemaker. It’s classified as a diamine, so you can expect some level of corrosiveness. The formula signals you to treat it carefully and check the safety data sheet before you even pop the cap. That’s a habit most chemists pick up quickly—nobody wants a chemical burn.
High school students memorizing formulas often wonder if the effort pays off. Here’s the case where it does. Knowing exactly what C4H10N2 represents means you can discuss 1-Aminopyrrolidine’s properties, its possible uses, and even brainstorm safer handling practices. If you’re part of a research team or a manufacturing group, being familiar with formulas shaves hours off troubleshooting and lets you choose the right compounds fast.
Companies could invest more in training new lab workers to look past formulas as just numbers and letters. They could build interactive guides where typing in C4H10N2 instantly shows you key info—hazards, usage, and disposal instructions. Another approach—chemistry teachers can link formulas to vivid stories or real products. After all, the formula of 1-Aminopyrrolidine sits as a link between lab techniques, safety, classroom fundamentals, and the search for better medicines.
1-Aminopyrrolidine doesn’t show up all over the news, but people working in chemistry or pharmaceuticals bump into it quite a bit. I’ve worked in a few labs where this compound played a quiet but important role. It’s not flashy, just a small molecule with a ring of four carbons and a nitrogen poking off, but its structure gives it a kind of flexibility, meaning chemists can use it in a range of reactions. Most people outside the field never hear about it, even though plenty of household products come from molecules built using these building blocks.
Drug makers value 1-Aminopyrrolidine for its usefulness as a starting material or intermediate. That means the molecule often goes through a few transformations before ending up in the final medicine. For instance, chemists use it to build more complex molecules that have biological activity—antivirals, antihistamines, and some types of painkillers trace their roots back to small steps involving 1-Aminopyrrolidine. In my work with a research team, we focused on tweaking the ring structure to change how a drug worked in the body; having a reliable supply of starting materials made all the difference.
Out on the farms, safer and more effective pesticides start with careful chemistry. Many modern pesticide molecules include cyclic amines—compounds built from structures like 1-Aminopyrrolidine—because they can interact specifically with the nervous systems of pests. I’ve sat in meetings discussing how to lower environmental impact, and much of the time, the answer lay in the details about what raw materials we fed into the process. Tossing something with the right size, shape, and bonding ability, like this compound, can help manufacturers create products that break down better and stick around for a shorter time in the soil.
For people tinkering with metalwork or oil, 1-Aminopyrrolidine helps keep rust at bay. Mixed into solutions that coat or clean metal pipes and parts, it slows the reactions that lead to rust. This isn’t the sort of chemistry that grabs headlines, but it’s essential. Chemical plants can run longer and safer when their equipment lasts, all thanks to these quiet molecules that never get much recognition.
With thousands of tons of chemical intermediates produced every year, keeping an eye on things like toxicity and waste always matters. Folks in the industry who deal with 1-Aminopyrrolidine have to pay attention to safe handling—exposure can cause skin and eye irritation, and nobody enjoys working with chemicals that have unclear long-term effects. Improving ventilation in workspaces, stricter gear rules, and more thorough training stand out as simple tools that protect workers. On top of that, green chemistry research could help uncover safer substitutes or find processes that need less of these compounds in the first place. The push for more sustainable chemicals comes down to creative thinking—and, often, hard work at the lab bench.
Walk into any chemistry lab and the periodic table never feels far away. In this ocean of molecules, 1-aminopyrrolidine offers its own style of intrigue. It belongs to the world of cyclic amines, where scientists and pharma professionals chase new promise for medicines and specialty chemicals. Each time I see the structure’s five-membered ring with a nitrogen at one corner and an amino group at another, I remember how small tweaks in an organic backbone can change the game for a researcher or a manufacturer.
For those who love numbers as much as names, 1-aminopyrrolidine carries the molecular formula C4H10N2. Pretty compact. Each atom brings weight to the table: carbon with 12 atomic units, hydrogen lighter at just 1, and nitrogen settled at 14. Punching those numbers in, the molecule’s total mass lands at 86.14 grams per mole. That sort of sum might look small, but for chemists, it means everything when measuring out reactants or scaling up in industry. I’ve watched reactions sink or soar simply because a calculation like this was botched or overlooked. Those small details tend to become big hurdles or happy shortcuts.
Molecular weight isn’t just trivia for a dusty textbook. In pharmaceutical labs, the right dose lists depend on these numbers. Too little, and the product stalls in clinical trials. Too much, and you could trigger side effects or extra costs. Different drugs need different calculations, but the math starts with knowing the weight of each player on the stage—including something as unassuming as 1-aminopyrrolidine.
Synthetic chemistry, which often juggles dozens of compounds at once, leans on these numbers in every batch. Fouling up a batch because the math felt like fine print can burn thousands of dollars in a single afternoon. Once I met a colleague who kept his own pocket “molar mass cheat sheet” to avoid costly mistakes—a habit that saved a project when a scale spat out one zero too many on a Friday night.
Beyond the lab, industries focus on waste. If one gram is out of place, kilograms multiply across production lines and the bills stack up. Regulatory agencies step in and check, too. Consistency only shows up if the little calculations—like the molecular weight of 1-aminopyrrolidine—don’t slip. Most people never see that side, but those who monitor quality control or batch documentation live in that world every day. I’ve lost count of rounds of paperwork spent proving every little atom in the mix adds up right.
Chemistry sometimes feels like a high-stakes recipe, and that’s not the kind of kitchen where anyone wants “a pinch more” or “a handful less.” When folks talk about making processes more efficient, the journey always comes back to clear, accurate numbers. Calculators don’t lie, but speed and overconfidence sometimes do. Refreshing training for junior staff, double-checking reference materials, and updating equipment can all help keep calculations sharp—and nothing smooths out workflow like nailing the molecular weight from the start.
So, the next time a conversation drifts toward scientific breakthroughs or quality slip-ups, chances are, somewhere along the line, someone checked their numbers and made sure that humble 86.14 didn’t trip up the team.
Ask anyone who has mixed chemicals in a lab, and they’ll tell you: some names start to earn extra respect after you handle them. 1-Aminopyrrolidine is one. With its sharp odor and tendency to irritate the eyes, skin, and lungs, it doesn’t belong in casual hands. Mixing, pouring, or storing it calls for serious attention. No matter if you spend every day around chemicals or if you’re just starting, herd instincts can lead to big problems when things go wrong—especially with a substance that releases dangerous fumes and can catch fire.
I’ve felt the sting of careless handling more than once—splashes, clouds of vapor, that little cough that follows you later. So, I never skip gloves or goggles around 1-Aminopyrrolidine. The risks aren’t worth bravado. Any skin exposure means painful irritation, so nitrile gloves and a lab coat block most of it. For eyes, even a tiny droplet stings, so wraparound goggles beat out simple safety glasses every time. Breathing in vapors leaves you with coughing fits—so, any time this compound leaves the bottle, I count on a chemical fume hood or, at the bare minimum, a tight-fitting respirator.
One thing folks sometimes forget: just because a chemical smells sharp doesn’t mean the danger stops with your nose. 1-Aminopyrrolidine vapor hangs in the air and can irritate lungs quickly. I’ve seen small, windowless rooms become a hazard zone fast. So, don’t trust your nose—trust a fan, fan-driven ventilation, or a working fume hood. No shortcut fixes what a proper exhaust setup delivers.
Chemicals with flammable vapors, like 1-Aminopyrrolidine, ask for strict habits. Keep it away from heat, sparks, and open flames. Once, I watched a careless spark turn a bench into chaos; the smell of burning plastic still lingers in my mind. Use containers made to hold solvents—never makeshift jars or bottles that could crack and leak. Check fire extinguishers before you start. Don’t just look at them once a year; make them a part of your setup routine every single time.
Spills tempt a quick, risky cleanup. Wrong move. Granular spill kits, meant for chemicals, beat paper towels by a landslide. A spill that soaks into towels can go straight through, risking skin and more fumes in the air. Sweep up the mess afterward, then wash your gloves off before tossing them. Dispose of waste in containers marked for hazardous chemicals—otherwise, the next person handling the trash gets an unpleasant surprise.
Nobody gets instinct for chemical safety overnight. Courses and experience build better habits than a label on a bottle can. Take the time to review safety data sheets. Run through spill drills. Some of the best lessons I’ve picked up came from older chemists and supervisors who saw danger coming before it showed up. Don’t just trust your memory—put checklists and signs where you work, so reminders stay front and center.The road to safe handling gets smoother with every careful habit and backup plan you build. The more people take 1-Aminopyrrolidine seriously, the fewer trips to the emergency room. That’s the big win nobody brags about, but it matters every day in every lab.
1-Aminopyrrolidine doesn't show up on most grocery lists. Still, I’ve seen many folks, even in skilled labs, let their guard down around chemicals that look less dangerous on paper. This one comes with risks—flammable, sometimes toxic, and known for its irritating vapors. No one wants a story that starts with a chemical leak and ends with a call to emergency services. That’s reason enough for stricter storage habits.
Years back, I worked alongside a chemist who never used fancy labels or sophisticated vaults, but his storage discipline saved us more than once. He’d pick a well-ventilated cabinet, lock away chemicals by type, and never let incompatible compounds get too cozy—especially ones like 1-Aminopyrrolidine. The careful separation from oxidizers, acids, and strong reducing agents made the biggest difference. Just a single careless shelf placement could turn routine storage into a flammable mess.
For temperature, room temperature in a cool, dry spot usually beats fancy refrigeration, unless a supplier’s sheet states otherwise. I’ve watched folks stuff everything into the fridge, thinking it’s the golden rule. Not always true. Humidity and heat raise the odds of spills, fumes, or worse. For people working in older buildings, keep an eye on insulation—small leaks in ventilation make a bigger impact over time.
One lab I visited suffered theft after leaving chemicals in plain sight. It might sound like overkill, but lockable cabinets protect everyone—not just from accidental use, but from people wanting to misuse solvents or reagents. Theft prevention gets overlooked, but it matters just as much as fire safety.
I run into questions about what happens if some liquid spills. Routine isn’t enough. Absorbent pads and neutralizers should always stay within arm’s reach. I’ve seen sloppy habits lead to vapors that send workers home sick. A strong local exhaust system and fume hood make a big difference. I bring this up because you can smell 1-Aminopyrrolidine’s sharp, unpleasant odor long before a monitor sets off an alarm.
Forget fancy charts and instead focus on immediate recognition. Label everything, even halfway-used bottles. No more mystery jars at the back of the shelf. Hazards don’t pause for confusion. I’ve lost count of stories where someone figured a small bottle was “just another amine” and regretted it seconds later.
It isn’t just about locking cabinets and slapping on hazard stickers, though. Keep everyone on the same page with regular reminders—it only takes one new employee to turn the routine upside down. Quick refresher sessions help. Sit down as a team, point at the labels, recall accidents, and remind each other of what burns or poisons. The best storage practices always come from lessons learned the hard way.
Regulations change, manufacturing tweaks, and safety data gets updated. Suppliers sometimes change packaging—sometimes use lighter plastics, sometimes glass. I always urge folks to check new shipments right away. If the MSDS or safety sheet flags any updates, review storage methods to keep in step. Ignoring manufacturer guidance or skip-reading regulatory advice leads straight into trouble.
Handling 1-Aminopyrrolidine isn’t rocket science, but it rewards respect and planning. Keep it cool and dry, double-check compatibility, and stay ready for the human error that always slips through. That’s what reliable storage really comes down to—persistence and a bit of old-fashioned caution.
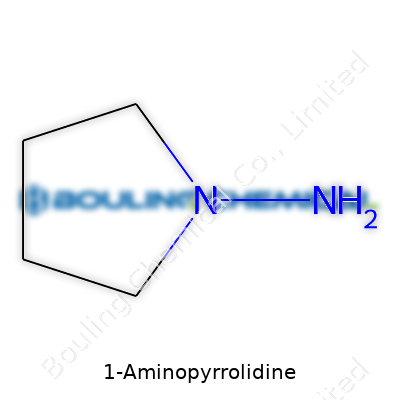
| Names | |
| Preferred IUPAC name | pyrrolidin-1-amine |
| Other names |
Pyrrolidin-1-amine 1-Pyrrolidinamine Aminopyrrolidine |
| Pronunciation | /waɪ-əˈmiːnoʊ-pɪˈrɒlɪdiːn/ |
| Identifiers | |
| CAS Number | 4280-62-0 |
| 3D model (JSmol) | ``` CC1CNCC1N ``` |
| Beilstein Reference | 2877585 |
| ChEBI | CHEBI:15940 |
| ChEMBL | CHEMBL149226 |
| ChemSpider | 85221 |
| DrugBank | DB04452 |
| ECHA InfoCard | ECHA InfoCard: 100.032.042 |
| EC Number | 211-670-0 |
| Gmelin Reference | 85249 |
| KEGG | C06284 |
| MeSH | D017214 |
| PubChem CID | 13506 |
| RTECS number | UQ9450000 |
| UNII | UO8QDW323H |
| UN number | UN2733 |
| Properties | |
| Chemical formula | C4H10N2 |
| Molar mass | **86.14 g/mol** |
| Appearance | Colorless to light yellow liquid |
| Odor | Ammonia-like |
| Density | 0.997 g/mL at 25 °C |
| Solubility in water | miscible |
| log P | -0.54 |
| Vapor pressure | 0.286 mmHg (25°C) |
| Acidity (pKa) | 10.5 |
| Basicity (pKb) | pKb = 3.16 |
| Magnetic susceptibility (χ) | -47.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.464 |
| Viscosity | 13 cP (25°C) |
| Dipole moment | 1.57 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 274.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -83.1 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3375.7 kJ/mol |
| Pharmacology | |
| ATC code | N04BC09 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin irritation, causes serious eye irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H311, H315, H319, H332, H335 |
| Precautionary statements | H315, H319, H335 |
| NFPA 704 (fire diamond) | 2-3-1 |
| Flash point | 77 °C |
| Autoignition temperature | 240 °C |
| Lethal dose or concentration | LD50 oral rat 2500 mg/kg |
| LD50 (median dose) | LD50 1000 mg/kg (rat, oral) |
| NIOSH | UR0115000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 200 – 800 mg/L |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
1-Pyrroline Pyrrolidine 2-Aminopyrrolidine Pyrrolidin-3-amine |