Chemists first took a closer look at pyrrole derivatives well over a century ago, right around the time organic chemistry blossomed as a serious field. As the world of pharmaceuticals began to open, odd little heterocycles like pyrroles attracted some attention for their quirky reactivity and biological roles. 1-Aminopyrrole didn’t take center stage at the start, but it spent time in the wings thanks to researchers exploring new nitrogen-rich compounds for dyes, drugs, and agrochemical leads. Labs with an eye for distinct molecular scaffolds pushed the study of simple aminated heterocycles, tracking down routes to 1-Aminopyrrole and its relatives. People learned the hard way about its tendency to oxidize and misbehave, yet these same headaches propelled innovation in protecting group chemistry and careful process design. Today, scientists see this molecule as both a building block, a synthetic challenge, and a potential key to unlocking new medicines or novel materials.
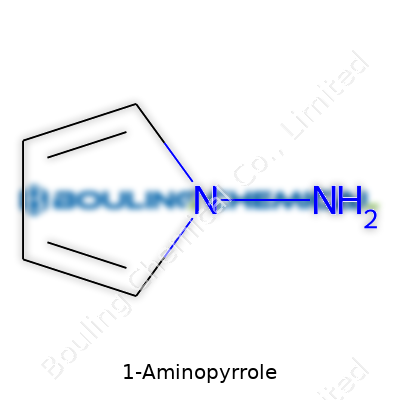
As a taut five-membered ring with both nitrogen and an amino group, 1-Aminopyrrole sits in the small but important family of aminated heterocycles. In the bottle, it usually comes as a pale, slightly yellow liquid, prone to browning if the seal isn’t tight and air breaks through. Its strong, pungent odor can fill the room during an opened experiment. This product sees primary use as an intermediate—almost nobody dabs it on final products without tweaking, but it often sits just one step away from something interesting. It lands in catalogs aimed at specialty chemical producers, medicinal chemists, and universities focused on synthesizing everything from complex natural products to new polymers.
On paper, 1-Aminopyrrole packs a punch: molecular formula C4H6N2, molar mass roughly 82.10 g/mol. It has a boiling point hovering near 160 °C, but watch out—with a low flash point, it asks for careful storage. In my experience, its water solubility surprises those expecting more hydrophobic behavior, showing modest mixing though never quite blending into aqueous solutions like a pure amine. Its lone pair on the ring nitrogen and a free amino group mean it lights up in typical nucleophilic reactions but also sits open to oxidation. In the lab, oxygen can turn fresh 1-Aminopyrrole brown in hours if left open. Analytical chemists mostly spot it by NMR, which displays well-resolved signals from the aromatic ring protons and the amino group.
A commercial sample gets shipped in amber vials to cut down on degradation, with clear hazard labels reflecting its sensitivity. MSDS usually flags its volatility and mild toxicity: gloves, goggles, and decent ventilation become non-negotiable. Catalog listings touch on its percent purity (typically above 97%) and note the batch’s water content as this can ruin many planned syntheses. Some suppliers certify it meets or beats analytical reagent standards, confirming identity with NMR, mass spec, and elemental analysis.
Older literature points to dauntingly indirect synthesis—starting with protected pyrrole derivatives, inching toward amination under harsh conditions, then peeling away groups and coaxing the precious amine into existence. The Bucherer–Bergs reaction and similar classical protocols let skilled hands transform pyrrole into the aminated ring, though yields swing wildly based on humidity and route. Recent years brought milder procedures, sometimes drawing on palladium-catalyzed amination or copper-mediated cross-couplings. These modern routes cut down on byproducts and save researchers from marathon purification slogs, which builds morale in any lab. As demand grows for greener chemistry, some groups work on one-pot methods that avoid toxic solvents, hinting at an easier path in the future.
You rarely find a chemist content with 1-Aminopyrrole as-is; people want to bolt on groups or protect the amine for later tricks. Its amino group serves as an anchor for acylations, sulfonylations, or carbamate protections, letting researchers walk it through multiple steps without falling apart. Electrophilic aromatic substitution can target the available positions on the ring, letting scientists stitch in halogens, nitros, or alkyl chains. Cross-coupling chemistry also shines: boronate chemistry, Suzuki couplings, and copper-catalyzed transformations enable a nearly endless array of new analogs. In my own work, small tweaks to the aminopyrrole core often shift solubility and reactivity, signaling just how sensitive the molecule is to minor changes.
Literature and catalogs juggle plenty of names, from 1-Aminopyrrole and pyrrol-1-ylamine to aminopyrrole. Occasionally, older references mention 1H-pyrrole-1-amine. No matter the label, double-checking the CAS Number—usually 4417-78-5—makes sure you don’t order the wrong cousin. Such synonyms can confuse those new to the field, but experienced chemists know to read below the headline in every catalog.
Handling 1-Aminopyrrole takes more than just care. Besides goggles and gloves, I always pair it with a fresh fume hood or airflow system. Skin contact can cause burns, and liquid easily seeps through latex if left unattended. Vapor exposure hits your throat with a sting. Spills stain benches and burn the nose, so clean-up kits and containment protocols become habits, not afterthoughts. Documentation from regulatory agencies flags its reactivity with oxidizers and possible mutagenic effects in high doses. Every institution keeps its own set of limits for storage, but nobody wants a stray bottle lingering in a warm storeroom. Waste collection follows the rules for amines and nitrogen-rich heterocycles, and many labs partner with certified disposal outfits as a matter of routine.
In pharmaceuticals, 1-Aminopyrrole lays groundwork for new leads, especially in oncology and central nervous system research. Medicinal chemists gravitate to it for the easy install of nucleophilic groups and the flexibility the ring imparts on binding fragments. Agrochemical development has used derivatives to probe enzyme targets in pests and plant pathogens. Material scientists sometimes wave it through their toolkits for building conductive polymers, aiming for everything from flexible electronics to new battery chemistries. I’ve seen research teams use it as an intermediate for macrocycles and peptidomimetics that mimic natural systems. Everyone working in these spaces recognizes that its quirks sometimes slow things down, but the payoff of getting an accessible amine on a pyrrole core still tempts teams to try—again and again.
Researchers, seeking to cut both costs and complexity, push for shorter routes and safer reagents in synthesizing 1-Aminopyrrole. Finding catalysts that handle atmospheric oxygen without blackening the sample sits high on many wish lists. In the literature, new papers arrive monthly, outlining updated protocols: biphasic extractions, enzyme-catalyzed aminations, and continuous-flow reactors all fight for the next step forward. Drug discovery programs see aminopyrrole scaffolds as points of departure for kinase inhibitors and epigenetic modulators, so high-throughput chemistry tools demand scalable and cleanly running 1-Aminopyrrole processes. Every small improvement in yield or purification recycles across research pipelines, feeding forward to speed up the hunt for a promising compound.
Compared to brute-force alkylating agents, 1-Aminopyrrole looks mild, but that doesn’t make it safe for the casual user. Animal studies reveal some hemolytic potential with high doses; mutagenicity screens yield conflicting results at the margins. Inhalation studies cause concern, especially after repeated exposure—headaches, nausea, and skin irritation pop up early. On the cellular level, its amine function can disrupt proteins or nucleic acids, triggering off-target interactions that don’t show up in early screeners. Regulators flag it for workplace monitoring, tying acceptable exposure limits to time and volume. My own cautious side—shaped by a few decades of solvent splashes and unexpected irritants—recommends keeping 1-Aminopyrrole on a tight leash, separating it from food, flame, and uninitiated co-workers. Emergency showers and eye wash stations matter far more with reactive amines like this than with most run-of-the-mill solvents.
The path ahead for 1-Aminopyrrole won’t disappoint those who like a challenge. Sustainable chemistry opens doors for new, safer routes. Automation and flow reactors hold out hope for minimizing error and waste, putting safer scale-ups within reach. Drug hunters continue to look for unique heterocycles to dodge resistance in cancer and infectious diseases; 1-Aminopyrrole derivatives sometimes spark enough activity to survive the first screen and move to later trials. Tech applications, from OLED materials to next-gen batteries, lean on the flexibility of this motif to bridge electronic and ionic domains. As more chemists aim for greener, cheaper, and less toxic synthetic methods, the toolkit around 1-Aminopyrrole expands. I expect to see new ligands, smarter protection strategies, and cross-disciplinary teams making the most of this occasionally tricky but deeply useful building block.
Stepping into any chemical research lab, I’ve learned that “purity” cushions every conversation around substances like 1-Aminopyrrole. Nobody trusts a bottle unless they know what’s really inside. This particular molecule shows up as a light yellow to brown solid, catching attention not for its looks but for its role in building more complex chemicals—sometimes in pharmaceuticals, other times in advanced materials. Purity throws a wrench into every experiment. If there’s even a little contamination—leftover solvents, water, or other pyrrole isomers—results skew, and scientists start cursing under their breath.
Most commercial batches promise upwards of 97% purity, with some specialty suppliers stretching to 98% or even 99%. Labs hoping to run kinetic studies or synthesize sensitive drugs get picky and chase the highest grades. A difference of one percent brings noticeable shifts in melting points, color, or even chemical smell. In several research stints, I’ve watched researchers debate whether a bottle’s stated 97% means they’ll spend double time purifying, or if they can risk jumping right in.
Impurities don’t just irritate the perfectionists—they wreck entire projects. Imagine sweating over a month of synthesis, only to figure out a trace impurity hijacked your catalyst or reacted with your test compounds. Chemical reactions run wild; yields plummet. For pharmaceutical uses, this gets dangerous. Medicines depend on strong purity standards to keep patients safe. The U.S. Pharmacopeia lays down strict numbers—usually 99% or better. Anything less calls for another round in the rotavap or distillation setup.
I’ve seen batches of 1-Aminopyrrole cloud up, hinting at water or oxidation byproducts. Some bottles show faint streaks of amber or a pungent whiff nobody trusts. Even with companies promising high purity, that’s judged by handful of standard tests—usually gas chromatography or HPLC. Not every impurity shows its face in those tests. Shadow contaminants, left over from how the chemical was made, pop up months later. That creates long-term headaches if anyone stores the stuff for future experiments.
Quality starts right at the factory. Cleaner synthetic routes cut down on leftover byproducts. Using fresh reagents, tight temperature controls, and avoiding exposure to air keep the product cleaner from the outset. In the lab, students and techs take things further, running extra recrystallizations or picking out purification methods like vacuum distillation. In my experience, double-checking new batches with in-house analysis saves trouble. If something looks off, nobody hesitates to reject a supplier or demand a refund.
Transparency between suppliers and customers makes a huge difference. Companies with detailed analytical data, even including those ugly impurity spikes, earn lasting business. Reliable suppliers don’t shy away from showing their lab reports, and that trust pays off down the line. Sterner regulations may help, but building good habits in the lab and honest communication keeps unsafe batches out of projects—and, especially, away from medicines.
1-Aminopyrrole isn’t just another chemical on the lab shelf. It has a way of reacting with air and moisture that can catch both new researchers and seasoned chemists off guard. My first encounter with pyrrole derivatives happened during an undergraduate summer internship. A small bottle sat in the fridge, wrapped tightly in foil, and my supervisor wasted no time explaining why.
Heat accelerates unwanted reactions. I remember opening a shipment of 1-Aminopyrrole left unintentionally at room temperature for too long. Even with just a few hours in a warm storeroom, the substance lost its punch and developed an unmistakable foul odor. Cool or even cold storage, ideally at 2–8°C, helps slow the decomposition. Most chemical catalogs echo this, since oxidation and polymerization move faster in warmth.
The bottle with the tightest seal usually holds up best. 1-Aminopyrrole oxidizes at a pace that can turn a pale, crystalline solid into a dark, useless mess. A well-sealed, airtight bottle keeps out oxygen and humidity. I’ve had colleagues who, out of habit, open and close their bottles carelessly. Opening it often, or forgetting to tighten the cap, spells disaster for the purity. Using containers made of glass or high-quality plastic, with screw caps lined with PTFE or another inert material, makes a strong barrier.
An old lab trick involves wrapping sensitive bottles in aluminum foil. Light, especially UV, pushes 1-Aminopyrrole into reactions that break it down or change its properties. Amber-colored bottles can help, but nothing beats storing the chemical in a dark place. Many researchers tuck it away in a closed cupboard or dedicate a drawer in a cold room.
Every container should carry a clear label with the date received and the last open date. In my own notes from grad school, I kept track of batch numbers, source, and any signs of change in appearance. Some labs go further, maintaining a log of how long a chemical has been open. This routine catches issues before a failed experiment exposes problems with storage.
Good storage is only half the story; safe handling matters just as much. 1-Aminopyrrole isn’t terribly toxic by organics standards, but I always glove up, work in a fume hood, and avoid breathing the dust. Clean spills right away, since even tiny amounts can stain or produce noticeable odors. Preventing skin contact keeps allergic reactions at bay.
One research group I knew missed contamination because they let condensation form inside a bottle brought from the fridge to the bench too quickly. Water ruins 1-Aminopyrrole; clumping or discoloration means water found its way in. The smarter approach brings the bottle to room temperature, still sealed, before opening.
Even smaller research outfits, with only basic refrigeration, can keep 1-Aminopyrrole viable for months by using small vials and transferring portions with clean, dry tools. For bigger setups, setting up a regular inventory check catches expiring stock and prevents accidental over-ordering. If your lab uses argon or nitrogen, storing particularly sensitive chemicals under an inert blanket stops air from creeping in, giving even longer shelf life.
Attention to storage details turns 1-Aminopyrrole from a temperamental liability into a reliable reagent. The checklist isn’t complicated—cold, sealed, dry, and dark. These routines don’t just save money; they keep experiments and reputations intact.
Interest in substances like 1-aminopyrrole has grown. Folks in research labs or specialized industries might turn to this material when searching for new building blocks for synthesis or novel pharmaceutical projects. The hitch—with this and plenty of other lab chemicals—is that access doesn’t always line up with demand. My experience poking around chemical catalogs or seeking quotes from big suppliers reflects that you can’t treat 1-aminopyrrole like sodium chloride. It pops up in offerings from select manufacturers, but not with the usual convenience shoppers expect from standard stock.
Whenever I’ve tried to order sensitive molecules, the catalog usually sorts them into categories like “research grade” or “technical grade.” High-purity research grade is what universities or pharmaceutical firms go for. Labs want confidence in their data, so cutting corners with impure batches leads to headaches down the line—contaminated reactions, faulty results, wasted cash. Some vendors do provide technical grades, mainly targeting those in early-phase material screening, where hitting analytical benchmarks isn’t make-or-break. From what I’ve seen, though, 1-aminopyrrole nearly always appears as a specialty item, and premium purity dominates, if it’s available at all.
Bulk drums of 1-aminopyrrole don’t move off shelves. Instead, you’ll see listings for tiny bottles—hundreds of milligrams, sometimes a gram or two. The reasons circle back to usage: synthetic chemistry tends to need only small amounts, since these reactions run on scales below what most people picture. Some chemists get used to this stop-and-go hunting for obscure materials, piecing together projects with what’s in reach, and adapting when a supplier drops a compound from their catalog.
The price tag for a gram can surprise newcomers. Specialty compounds like 1-aminopyrrole carry the markup of custom work. Companies producing it must manage hazards and specialized equipment—a far cry from commodity chemicals. Add in Customs paperwork and local chemical regulations, and the hurdles multiply. I’ve run into months-long waits for shipments, especially if sourcing from overseas suppliers. Delays strain progress, spike costs, and often discourage smaller labs from exploring less common molecules.
It helps to talk to chemical suppliers early. Some companies will produce 1-aminopyrrole on demand, but only once they’ve seen enough interest. People in research and industry can push for better access by pooling requirements or flagging needs to purchasing consortia. I’ve seen university networks organize bulk orders, cutting costs and smoothing logistics. For small companies, custom synthesis services step in, provided your budget stretches far enough. It pays to double-check safety documentation, too—handling chemicals like 1-aminopyrrole safely isn’t optional, and supplier guidelines don’t always match local standards.
Making specialty materials easier to obtain matters for anyone hoping to push innovation. If more suppliers hear the call for reliable small-batch options and support with both regulatory paperwork and prompt delivery, market access widens. Until then, working with 1-aminopyrrole remains a lesson in patience, careful planning, and sometimes creative workaround.
Walk into any research lab playing around with heterocycles, and 1-aminopyrrole isn’t going to be the flashiest bottle on the shelf. It doesn’t draw the same headlines as, say, graphene or CRISPR. What it does is quietly open possibilities in both medicinal and materials chemistry, showing up in places that matter more often than people realize.
Almost every time someone chases a new drug for cancer or infectious disease, they reach for nitrogen-rich heterocycles. 1-Aminopyrrole brings a unique twist to these molecules, putting a reactive amino group right onto the pyrrole ring. Medicinal chemists see clear opportunities here, not only because of the reactivity, but because this arrangement often helps the resulting compounds slip inside cells or interact tightly with enzymes.
For example, several researchers working on kinase inhibitors for cancer have built their scaffolds using aminopyrroles. The basic structure offers the kind of hydrogen-bonding and stacking properties that protein-targeting drugs rely on. Some antifungal drugs explored over the last decade also include this core. In my own early days at grad school, I watched a colleague struggle to tweak a fused aminopyrrole structure to get better action against bacterial enzymes — and when it worked, the improvement wasn’t small. That showed me how a subtle structural change, like adding an amine to a pyrrole, can turn the tide in medicinal programs.
The excitement around polymers often focuses on conductivity and flexibility, with everyone wanting to make new screens, batteries, and sensors. Here, 1-aminopyrrole starts to show another side. It serves as a building block for polypyrroles, a family of electrically conductive polymers. Add that amine group, and suddenly, chemists have a way to shape the reactivity, solubility, and electrical properties of the finished materials.
Researchers in organic electronics have managed to tune the charge carrying ability of their polymer films by dropping in these aminated structures. They get better adhesion to surfaces, more responsive sensors, and even new colors for organic LEDs. Anyone following the rise of cheap solar cells has seen these tweaks lead to better power conversion rates. Not every fan of gadgetry knows it, but the science behind their brighter, longer-lasting displays often has roots in molecules like 1-aminopyrrole.
Synthetic chemists always hunt for ways to construct complex architectures more efficiently. Aminopyrroles slip in as 'privileged scaffolds’ —they show up in countless reaction schemes, from Suzuki couplings to cyclizations headed for macrocyclic drugs or advanced dyes. I remember talking to a synthetic chemistry professor who said aminopyrroles saved him days in lab, all because they handled functional group tolerance better than most similar compounds.
It’s not just about flexibility: the presence of both the amino and pyrrole groups means researchers can hook new fragments on with minimal fuss. This trait sparks creativity, whether the goal is a next-gen pigment or a better inhibitor for an enzyme. Even graduate students chasing their next publication have found new applications just by thinking up new combinations with aminopyrroles.
Aminopyrroles can be a little tricky to synthesize and handle, reacting with air or moisture in ways that frustrate scale-up. Some research teams work around this by using in situ generation — they make aminopyrrole right during the reaction instead of isolating the pure chemical. There’s been progress in stabilizing derivatives, too, opening the door to broader use.
On the safety front, proper handling matters. Like most aromatic amines, they demand gloves and good ventilation. Advances in green chemistry could someday make their production both safer and more sustainable, a shift the entire field could benefit from.
You don’t spot 1-Aminopyrrole on the shelf next to baking soda, and for good reason. This compound belongs to a family of chemicals that can turn a routine day in the lab into one filled with stories you don’t want to tell. Anyone who has handled nitrogen-containing heterocycles understands that safety isn’t a box to tick. It’s the difference between uneventful experimentation and a real hazard.
My early days in the lab taught me that even a few drops can change everything. 1-Aminopyrrole releases vapors rapidly and can catch fire at a spark’s suggestion. Flash points for similar compounds regularly fall below room temperature. Open flames, static sparks, and hot plates need a respectful distance. Fume hoods quickly shift from “nice-to-have” to “must-have” to avoid breathing in fumes or risking ignition.
Splashing 1-Aminopyrrole tells you, in the worst way, that basic gloves don’t always cut it. Nitrile or neoprene gloves gave me peace of mind after one close call. Simple latex ones seemed to melt or allow skin irritation. Splash goggles and lab coats turned into a daily uniform, not just a formality. Hard, acrylic shields sometimes step in for added insurance during transfers.
Without a proper fume hood, the sharp, disagreeable smell of pyrroles becomes more than an annoyance. Irritated eyes, headaches, and throats remind you that inhaling isn’t trivial. Fans and open windows suppose to help, but enclosed hoods do the serious work. I always make sure there’s a working extraction fan, especially during synthesis or dilution.
Too many accidents start with a misread bottle or a forgotten label. Freshly distilled 1-Aminopyrrole goes straight into airtight, amber bottles. Light breaks down compounds faster than expected and can even spark off unwanted reactions. Stash it away in a flammable materials cabinet, away from acids and oxidizers. After a leak in my first research job, I invested in spill trays and secondary containers. It seems extra, until you clean up after a toppled bottle.
Speed matters if a spill happens, but panic turns a mess into a disaster. Absorbent pads, sand, or vermiculite stop the spread. I never grab water since some materials react unpredictably. Standard lab waste bins don’t cut it—hazardous waste rules apply. I learned to keep a class B fire extinguisher within arm’s reach and never leave the lab after a spill, even if someone promises to “watch it.”
No piece of paper covers real readiness like actual drills. Regular rehearsals for evacuation, eyewash use, and spill response made me quicker and calmer when things almost went south. Keeping up with new safety sheets and talking through procedures with coworkers fostered a safer work culture, and you could see the confidence during emergencies.
Manufacturers continue refining packaging, aiming for better seals and clearer warning labels. I push for digital inventory management, with expiration reminders, automatic supply checks, and direct links to up-to-date MSD sheets. It’s a game-changer to catch old or degraded chemicals before they become a problem.
| Names | |
| Preferred IUPAC name | 1H-pyrrol-1-amine |
| Other names |
1-Pyrrolylamine Pyrrol-1-ylamine |
| Pronunciation | /waɪˈæmɪnoʊˌpɪroʊl/ |
| Identifiers | |
| CAS Number | 5192-43-4 |
| Beilstein Reference | 120924 |
| ChEBI | CHEBI:36660 |
| ChEMBL | CHEMBL3214792 |
| ChemSpider | 129193 |
| DrugBank | DB08373 |
| ECHA InfoCard | 100.016.703 |
| EC Number | 1.4.3.4 |
| Gmelin Reference | 9715 |
| KEGG | C18613 |
| MeSH | D017246 |
| PubChem CID | 13515 |
| RTECS number | U2930-97-5 |
| UNII | 9T8C3146SV |
| UN number | UN2736 |
| Properties | |
| Chemical formula | C4H6N2 |
| Molar mass | 68.09 g/mol |
| Appearance | Light brown solid |
| Odor | amine-like |
| Density | 1.06 g/cm³ |
| Solubility in water | slightly soluble |
| log P | 0.02 |
| Vapor pressure | 1 mmHg (20°C) |
| Acidity (pKa) | 17.0 |
| Basicity (pKb) | 5.95 |
| Refractive index (nD) | 1.570 |
| Viscosity | 0.956 cP (25°C) |
| Dipole moment | 1.96 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 143.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -23.5 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -550.9 kJ/mol |
| Hazards | |
| GHS labelling | GHS02,GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H312, H332 |
| Precautionary statements | P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 74 °C |
| Autoignition temperature | 315 °C |
| Explosive limits | Explosive limits: 2.2–10.7% |
| Lethal dose or concentration | LD50 (oral, rat): 640 mg/kg |
| LD50 (median dose) | LD50 (median dose) of 1-Aminopyrrole: 50 mg/kg (mouse, intravenous) |
| NIOSH | Not established |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 1-Aminopyrrole is not specifically established by OSHA. |
| REL (Recommended) | 100 mg |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Pyrrole 2-Aminopyrrole Pyrrolidine Pyrrole-2-carboxylic acid Indole |