Interest in 1-Amino-1H-Pyrrole-2-Carbonitrile picked up as organic chemistry turned toward heterocyclic compounds in the twentieth century. Chemists looked for new building blocks in medicinal chemistry and advanced materials. Research from the 1960s and beyond explores the basic pyrrole skeleton, with groups adding functional elements—like nitriles—to push boundaries in pharmaceuticals and dyes. Over time, each milestone in synthesis or application often comes from researchers seeking higher yields or improved selectivity. They often face issues with reactivity and side products, which drives iterative improvement in laboratory technique and process scale-up.
1-Amino-1H-Pyrrole-2-Carbonitrile sits at an intersection between basic organic research and targeted product development. The molecule itself—a five-membered ring equipped with both amino and nitrile groups—brings unique reactivity. Laboratories favor it as a core intermediate, especially where the aim involves constructing more complex pharmaceuticals or dyes. Its structure allows for multiple transformation routes, making it a flexible tool among synthetic chemists. The commercial availability reflects growing demand, and today you can find this compound from most scientific suppliers who serve the pharmaceutical, agrochemical, and research sectors.
In its pure form, 1-Amino-1H-Pyrrole-2-Carbonitrile usually looks like a pale to off-white solid. It weighs in at about 109 g per mole, which keeps it manageable in a small vial or batch reactor. Solubility trends show moderate preference for polar organic solvents—think dimethyl sulfoxide or methanol—though it holds relatively stable in water, especially at neutral pH. The nitrile and amino groups give it a sharp melting point that anyone running purification or melting tests will appreciate when tracking impurities. Chemically, the position of the amino group next to the heterocyclic nitrogen tends to tune the basicity and makes nucleophilic attacks more selective than you'd find in a simpler pyrrole.
Suppliers typically package this compound with an assay report, often above 98% purity by HPLC or NMR. You’ll get the batch number, synthesis lot, storage recommendations—usually cool, dry, and away from strong oxidizers—and the chemical structure on the label. Most safety data sheets flag both the acrid-like odor and potential respiratory hazards during handling, which I’ve noticed helps labs enforce good ventilation and glove use. From what I’ve seen, the best-run facilities keep the compound in clearly labeled vials with hazard icons for eye, skin, and respiratory irritation. Alongside, manufacturers issue documentation on shelf life, typically two years if stored correctly.
Synthesis often starts from commercially available 1H-pyrrole-2-carbonitrile. Amination proceeds under mild to moderate conditions, usually in the presence of an amine source such as ammonia or ammonium salts, with catalysts like palladium or transition metal complexes assisting the transformation. Some academic groups streamline this route, coupling both steps into a single-pot synthesis with little need for extensive purification. I’ve followed studies where microwave or ultrasound activation sharply cuts down reaction time and boosts yield. Waste management matters here, so spend time planning solvent recovery and minimizing ammonia discharge into water streams.
Chemists rely on this core to build more elaborate molecules. The amino group works as an entry point for further functionalization: acylation leads straight into peptide-like structures, while diazotization opens the door to aryl or halogen substituents at the 1-position. The nitrile moiety supports nucleophilic addition and cyclization, important for expanding into fused-ring systems with bioactivity. I’ve observed labs use metal-catalyzed cross-coupling—Suzuki or Sonogashira—to create diversity around the ring, which helps unlock new hit compounds for pharma pipelines. The ability to selectively modify the scaffold gives this chemical much of its research value.
Different catalogues may list this molecule under several synonyms such as 1-Amino-2-cyanopyrrole, 2-Cyano-1-aminopyrrole, or 1H-Pyrrole-2-carbonitrile, 1-amino-. These labels trace back to variations in IUPAC and CAS naming, which can trip up anyone cross-referencing databases or searching patent literature. Commercial suppliers might favor simplified names or trade tags for cataloging, and researchers often abbreviate it to ACPN or related shorthand in notebooks and publications. Keeping track of these names matters for compliance, storage, and avoiding ordering errors.
Handling this molecule comes with the standard precautions of organic synthesis. Nitrile- and amine-containing organics tend to irritate the skin, lungs, and eyes on contact. Safety sheets recommend gloves, goggles, and working under a chemical hood, especially if heating or using large batches. Proper labeling reduces exposure risk; I’ve caught more than one incident in a teaching lab where someone unscrewed a container without checking hazard codes. For fire safety, it helps to know that the compound combusts to release potentially toxic nitrogen oxides and hydrogen cyanide, pushing teams to keep water and CO2 extinguishers on hand. Spill kits—absorbent pads and neutralizing agents—make for fast cleanup and maintenance of safe workspaces.
Pharmaceutical research uses 1-Amino-1H-Pyrrole-2-Carbonitrile to create kinase inhibitors, antiviral candidates, and anti-inflammatory scaffolds. The rapidly tunable ring system drives it into the lead optimization stage when fine-tuning binding properties for drug targets. Chemical manufacturers draw on this core to design functional dyes, especially those that demand thermal stability and electronic versatility. Agrochemical development benefits from its reactivity, converting it into key intermediates for fungicide and herbicide pipelines. These practical uses depend on ready access to both small and bulk batches, each with precise purity specifications and documentation.
Over the past decade, research teams have deepened their focus on this compound’s potential in both medicinal chemistry and material science. Machine-aided drug design programs frequently select pyrrole-based scaffolds as starting points, often leading to partnerships with suppliers for larger sample runs. Collaboration between academia and industry speeds up trial of new reaction conditions, catalysis, or green chemistry adaptations—especially useful for regulatory compliance and waste management. Many labs now publish both synthetic routes and biological data, making the pathway from lab-scale synthesis to preclinical trials more transparent. Regular method improvements show up at conferences where chemists share insights, often crediting minor tweaks in protocol with major gains in yield or product selectivity.
Much of the early toxicity data on pyrroles covers basic acute effects: skin redness, eye irritation, and inhalation hazards. More recent research digs into metabolic pathways, especially with regulatory agencies requiring chronic exposure studies for pharmaceutical and agrochemical candidates. Animal studies indicate moderate to low oral toxicity, but the compound’s nitrile content flags some caution—hydrolysis could release cyanide under certain biological conditions. I’ve seen in vitro tests focusing on genetic toxicity, with some reports suggesting low mutagenicity, though more investigation happens anytime the core gets included as part of a drug nucleus or agricultural product. Full risk assessments depend on intended use, so toxicologists collaborate closely with process chemists during product development.
Demand continues to grow for heterocyclic building blocks, especially with the steady expansion of pharmaceutical and advanced materials markets. Researchers are working on cleaner and safer synthesis methods, with interest shifting toward sustainable routes—biocatalysis, recyclable solvents, greener transition-metal catalysts. Improvements in process chemistry reduce both cost and environmental impact, making large-scale use more viable. Applications in biologically active molecules remain the main driver, as screening programs find new uses for functionalized pyrroles in infectious disease, cancer, and neurodegeneration research. As machine learning accelerates compound design, chemists will lean even more on versatile intermediates like 1-Amino-1H-Pyrrole-2-Carbonitrile to keep pace with fast-changing innovation cycles. Industry partnerships, clear labeling, and high regulatory standards shape how these molecules move from benchtop curiosity to mainstream application. Experiments in my own experience highlight the importance of cross-disciplinary teams—safety, synthetic skill, and real-world need all in action—to unlock the chemical’s promise.
1-Amino-1H-pyrrole-2-carbonitrile doesn’t turn many heads outside of research labs, but it plays a more meaningful role than its name suggests. It’s a small organic molecule, sort of like a toolkit for chemists looking to build much larger, more complicated compounds. If you’ve ever visited a pharmaceutical research lab or talked with someone deep into organic chemistry, they’d tell you about compounds like this. They’re the stepping stones for a whole range of new medicines and chemical products.
Pharmaceutical innovation relies on building blocks that offer versatility and reactivity. 1-Amino-1H-pyrrole-2-carbonitrile fits that bill perfectly. Researchers reach for it while working on new drug candidates because the aminopyrrole structure can be shaped into a wide range of bioactive molecules. For instance, various cancer-fighting agents, anti-inflammatory drugs, and antiviral compounds used in experimental stages of drug development trace their origins back to small, adaptable starting materials like this one.
Speaking from my own experience working around synthesis chemists, I’ve seen compounds like this used almost like raw lumber in a woodshop. They form the backbone for new molecular prototypes. These prototypes undergo rounds of testing to look for activity against disease targets, and if they show promise, chemists adjust the structure a bit to boost effectiveness or reduce side effects. Without flexible, reactive building blocks like 1-Amino-1H-pyrrole-2-carbonitrile, much of this work would hit a roadblock before it ever started.
This compound has importance outside pharmaceuticals, too. In specialty chemicals, scientists look for reliable starting points to synthesize dyes, pigments, and agrochemical intermediates. The aminopyrrole fragment serves as a bridge to build further complexity into molecules that might find their way into agricultural products or high-value specialty coatings. My conversations with colleagues working in applied chemistry have highlighted how much they value substances that can lead to quick, clean reactions—this one stands out in that category.
As customers and workers learn more about chemical safety, nobody wants surprises. Research labs and manufacturers need to understand the risks and safe-handling procedures for substances they work with. 1-Amino-1H-pyrrole-2-carbonitrile requires careful handling: it isn’t something to store next to your household cleaning supplies. The typical lab or factory staff checks the material safety data sheet, wears gloves, and keeps processing under good ventilation—routine, yes, but vital. Responsible research also looks at managing waste and limiting accidental release into the environment. Regulatory checks in major economies require scientists and producers to study and report any significant hazards, which helps safeguard workers and the nearby community.
Organic chemistry thrives when it keeps refining tools that let innovation happen faster and with fewer unwanted side effects. With sustainability growing as a priority, chemists look for alternatives that are both effective and safer for humans and the planet. That means green chemistry and better waste management join the list of priorities. Companies and labs experiment with processes that use less solvent, lower energy, and reduce hazardous byproducts—even with chemicals like 1-Amino-1H-pyrrole-2-carbonitrile, which already has a reputation for being fairly reliable. As research evolves, so do expectations for efficiency, safety, and responsibility—both in the lab and afterwards.
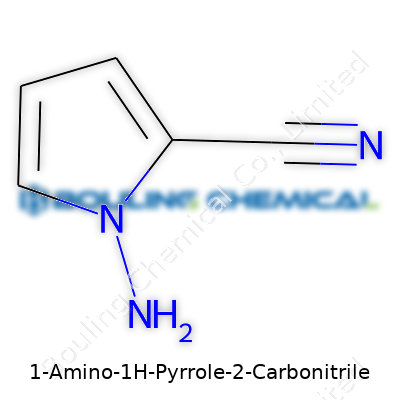
Exploring the nature of 1-Amino-1H-pyrrole-2-carbonitrile means diving straight into molecular structure. The chemical formula for this compound is C5H5N3. This formula doesn’t just show a set of numbers and letters—it represents the precise count and kind of atoms binding together to create its distinct properties. Five carbon atoms, five hydrogen, and three nitrogen atoms all shape its unique behavior in chemical reactions.
Molecular weight serves as a key touchstone for chemists, pharmacologists, and enthusiastic students checking reaction balances or planning syntheses. For 1-Amino-1H-pyrrole-2-carbonitrile, add up the atomic masses of each element: carbon (about 12.01 g/mol), hydrogen (1.008 g/mol), and nitrogen (14.01 g/mol).
Let’s work it out:
Total = 60.05 + 5.04 + 42.03 = 107.12 g/mol
Working in labs for years, simple details like a chemical formula or molecular weight help save both time and resources. Synthesizing a compound gets risky without knowing its building blocks. The wrong measurements mean wasted batches, extra costs, and sometimes hazardous outcomes. That’s more than just a hassle—it’s a matter of lab safety. When I worked as a research assistant, every new synthetic procedure began with these basics written clearly on the lab bench for easy checks. No step skipped. No numbers guessed.
1-Amino-1H-pyrrole-2-carbonitrile, by virtue of its amine and nitrile groups, walks with promise toward medicinal and material chemistry. Its structure allows it to join in diverse reactions, making it a potential building block in the design of larger, bioactive molecules. Pharmaceutical teams studying small molecule inhibitors, for example, focus on compounds like this because functional groups on such molecules provide spots for adding or tweaking desirable biological activities.
Researchers and manufacturers also track exact molecular weights for another vital reason: purification and analysis. High-Performance Liquid Chromatography (HPLC), Mass Spectrometry, and Nuclear Magnetic Resonance (NMR) all demand correct inputs for analysis software. Input the wrong numbers, and peaks on a spectrum become confusing, risking errors in publication or costly recalls in scale-up situations. Correct chemical identity and stoichiometry don’t just ease research—they assure compliance with regulatory standards.
Plenty of accidents and recalls happen because someone wrote down the wrong formula or skipped a basic calculation. Rooting scientific work in these fundamentals stands as a first defense. Clear labeling, double-checks, and routine calculations form the backbone of safe and efficient chemical development. Supporting new chemists and students to treat these elemental pieces with seriousness helps guard both scientific progress and personal safety. The goal is simple: fewer surprises, smoother progress, and reliable results in every bottle and beaker.
Working with chemicals like 1-Amino-1H-Pyrrole-2-Carbonitrile brings a challenge many overlook. One gets used to storing common solvents or buffers on a familiar bench rack, but specialty organics demand more foresight. This compound doesn’t behave like dry sugar or table salt; its nitrogen groups and the pyrrole ring don’t play nice with air, moisture, or careless handling. In my early lab days, I made the mistake of underestimating air-sensitive chemicals, leaving a few grams of a similar pyrrole overnight only to find it changed color and barely produced a valid spectrum. Lesson learned.
This is a solid, so one skips thinking about leaks, but the hazards—moisture uptake, light sensitivity, and potential for air oxidation—stay real. Locked drawers at room temperature won’t cut it. Dry, airtight containers work better, preferably in a desiccator cabinet or well-sealed bottle with a solid rubber stopper. Throwing a little silica gel pack inside keeps humidity at bay, something I never skip with pyrroles or cyanides. Never underestimate the smell; even small spills can leave a noticeable odor, and catching wafts reminds those of us in shared labs to use secondary containment—a simple plastic box with a snap-lid offers a low-tech but effective solution.
Cool and dark make a reliable mantra. Chemicals like this can degrade faster under sunlight. Cooler temperatures slow down unwanted reactions; I know folks storing similar molecules in a standard fridge, but a flammable chemicals fridge works best for anything questionable. Of course, always label and segregate—no stacking this near food or biology samples.
Gloves are a must, and goggles too. Avoid skin and eye contact, as both the amine and nitrile groups bring their own risks, and sometimes gloves pick up odors. I keep a dedicated waste bottle for contaminated materials, labeled with every compound for easy pickup by safety staff. Fume hoods take the sting out of any manipulations, whether weighing out powder or dissolving it in solvent. I once saw a friend skip the hood and spend the afternoon scrubbing odor out of the benchtop—lessons like that teach more than any manual.
Hands-on work always gets safer with clear labeling. Even if it sounds excessive, I date every jar and add a printed MSDS in the storage area. Fast identification beats scrambling in an emergency. Data from Sigma-Aldrich and trusted supplier sheets mention the risks of inhalation and recommend keeping spill kits nearby, focusing on dry cleanup and prompt disposal. Between government chemical hygiene plans and experience from old-timers in the lab, consistent double-checking pays off.
Most issues start with shortcuts: Improper sealing, lazy labeling, or skipping safety goggles because “nothing ever happens.” Teaching young scientists to track inventory prevents forgotten bottles. Regular inspection for leaks, moisture, and container integrity takes ten minutes but prevents days of cleaning up messes. Even in the busiest research groups, making safety part of daily routines saves trouble down the road.
People might not bump into 1-Amino-1H-Pyrrole-2-Carbonitrile at the grocery store, but it matters to folks working with chemicals or even studying new pharmaceuticals. Chemistry brings innovation, yet it always walks alongside the question of safety. This compound, a niche building block for science and industry, raises real concerns around toxicity because its structure contains groups—like nitrile and amino-pyrrole—that often lead to trouble for living things.
The world keeps learning from past mistakes. Aromatic amines and nitrile-bearing substances historically have triggered alarms. Many, like acetonitrile and certain aminopyrroles, are linked to harmful effects in humans or animals. Nitrile groups sometimes break down in the body to release cyanide, a notorious cellular killer. This is no small risk. The structure here, with its reactive groups, raises valid eyebrows about possible acute poisoning or slow, creeping damage to organs.
Detailed studies on 1-Amino-1H-Pyrrole-2-Carbonitrile are tough to find, but wisdom suggests staying cautious. Safety datasheets for similar chemicals warn about skin and eye burns, lung irritation, and gut problems if swallowed. The chemistry matches. Exposure, even at low levels over time, can cause trouble that sometimes gets mistaken for other health issues until it’s too late. Workers in research and manufacturing shouldn’t play dice with their lungs or liver.
Most people probably encounter threads of news relating to chemical spills or workplace exposure instead of this specific compound. Labs and factories use small or large quantities, often in closed systems. The slip-ups usually come from accidents, poor handling, or inadequate training. Once something hits the air or sticks to workers’ gloves, it can jump the containment and run wild in the environment or the bloodstream.
Smart companies already treat untested chemicals as threats unless proven safe. Following OSHA or EU REACH rules can offer breathing room, literally and legally. Gloves, goggles, fume hoods, and training shouldn’t just gather dust; these become the front lines for defending health. Regular monitoring, honest labeling, and updating protocols matter as much as any new discovery in the lab.
Clear labeling and open communication between chemists, managers, and safety officers help people handle 1-Amino-1H-Pyrrole-2-Carbonitrile with fewer surprises. Medical professionals also need to know what someone’s handling to spot symptoms early. Good companies don’t hide hazards; they face them head-on, providing access to Material Safety Data Sheets (MSDS), setting up spill response plans, and keeping emergency equipment in working order.
Funding more research on chemicals like this can clear up the picture. Universities and industry should pitch in to run animal tests, cell studies, or even human epidemiological studies (with consent) to get real risk data. Sharing findings publicly, instead of locking them away, lets everyone make smarter decisions, from government inspectors to people wearing lab coats in a dusty corner of a research park.
Anyone who’s ever worked in a lab, or dabbled with organic synthesis, has run into one simple truth: purity shapes results. For 1-Amino-1H-pyrrole-2-carbonitrile, the typical purity level touted by suppliers runs at 97% or above. In chemical terms, that might not sound like a big deal, but ±3% can mean the difference between a clean reaction and a soupy mess of byproducts. Some suppliers do offer purities reaching 98% or even the prized 99%, which starts to edge toward high-performance territory, usually required for pharmaceutical research or sensitive analytical work.
I’ve seen research teams breathing easy only because the batch of this compound hit their purity target; otherwise, purification steps steal precious time and resources. The small-print difference between 95% and 98% purity lands squarely on the bottom line. For new drug analog development or combinatorial chemistry, even minor impurities can gum up reactions, muddy results, and sometimes even risk equipment.
The President’s 2021 Council of Advisors on Science and Technology made it clear: better supply chains for reliable reagents fuel innovation. That’s not just bureaucracy speaking—repeatable, reliable outcomes rest on high purity starting materials like 1-Amino-1H-pyrrole-2-carbonitrile.
Bulk purchases don’t always make sense in research and niche manufacturing. Chemical companies typically offer this compound in an array of packaging sizes. Most common are 1-gram and 5-gram bottles, which suit academic labs and those doing small-scale synthesis. Larger sizes such as 25-gram, 50-gram, and even 100-gram units pop up for groups scaling up a project or running pilot batches.
I recall plenty of times opening a 5-gram vial, knowing it would save me countless headaches down the line. Too much product sitting on the shelf risks degradation, and that’s pure waste. Too little, and you’re chasing backorders and blown project timelines. Most labs don’t want drums of a specialty heterocycle collecting dust, but those expanding to small commercial scale sometimes need that 100-gram jar.
Flexible packaging meets project needs while managing costs and compliance. With narcotic precursors and obscure intermediates, suppliers also shape their packaging to match regulatory hurdles, shipping restrictions, and practical handling.
A scientist, procurement officer, or project manager navigating this space can lean on a few best practices. Ask for certificates of analysis and batch traceability as a baseline—especially if you’re scaling anything with regulatory implications. Always check stability and storage recommendations, as small batches may be your friend in the long run.
Big suppliers might not budge much on packaging, but specialty resellers sometimes accommodate custom sizes. Open lines of communication with suppliers, and combine orders where possible to streamline acquisition. It pays to compare sources, especially for significant differences in purity.
One thing sticks with me after years in labs: buying the right amount at the right purity level keeps teams focused on chemistry, not troubleshooting. In the end, purity and size drive more than convenience—they shape safety, savings, and scientific progress.
| Names | |
| Preferred IUPAC name | 2-Cyano-1H-pyrrol-1-amine |
| Other names |
1-Amino-2-cyanopyrrole 2-Cyano-1-aminopyrrole 1H-Pyrrole-2-carbonitrile, 1-amino- 2-Pyrrolecarbonitrile, 1-amino- |
| Pronunciation | /waɪˈæmɪnoʊ wʌn eɪtʃ paɪˈroʊl tuː kɑrˈboʊnaɪtraɪl/ |
| Identifiers | |
| CAS Number | 14110-30-6 |
| 3D model (JSmol) | `3D model (JSmol)` string for **1-Amino-1H-pyrrole-2-carbonitrile**: ``` CC1=CC(=NN1)C#N ``` |
| Beilstein Reference | 120990 |
| ChEBI | CHEBI:158284 |
| ChEMBL | CHEMBL1871594 |
| ChemSpider | 12795113 |
| DrugBank | DB08574 |
| ECHA InfoCard | 08e7f3cf-1a7c-45ed-b0e3-cf9c3056a832 |
| EC Number | EC 695-691-7 |
| Gmelin Reference | 837864 |
| KEGG | C18954 |
| MeSH | D000587 |
| PubChem CID | 11263277 |
| RTECS number | UX4375000 |
| UNII | X2P386U0GS |
| UN number | UN3439 |
| Properties | |
| Chemical formula | C5H5N3 |
| Molar mass | 107.12 g/mol |
| Appearance | Light brown solid |
| Odor | Odorless |
| Density | 1.16 g/cm3 |
| Solubility in water | Slightly soluble in water |
| log P | -0.52 |
| Vapor pressure | 0.00974 mmHg at 25°C |
| Acidity (pKa) | pKa = 1.89 |
| Basicity (pKb) | 3.41 |
| Refractive index (nD) | 1.580 |
| Dipole moment | 3.85 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 157.3 J/mol·K |
| Std enthalpy of formation (ΔfH⦵298) | 224.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -452.8 kJ·mol⁻¹ |
| Hazards | |
| Main hazards | Hazardous if swallowed, inhaled or absorbed through skin. Causes skin and eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-2-1 |
| Flash point | 120.7 °C |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 >2000 mg/kg |
| NIOSH | Unknown |
| REL (Recommended) | 0.05 mg/m³ |
| Related compounds | |
| Related compounds |
1-Acetyl-1H-pyrrole-2-carbonitrile 1-Benzyl-1H-pyrrole-2-carbonitrile 1-Methyl-1H-pyrrole-2-carbonitrile 1H-Pyrrole-2-carbonitrile 1-Amino-4-methyl-1H-pyrrole-2-carbonitrile |